Safety and Efficacy of a Novel Vaginal Anti-infective, TOL-463, in the Treatment of Bacterial Vaginosis and Vulvovaginal Candidiasis: A Randomized, Single-blind, Phase 2, Controlled Trial
- PMID: 30184181
- PMCID: PMC6376090
- DOI: 10.1093/cid/ciy554
Safety and Efficacy of a Novel Vaginal Anti-infective, TOL-463, in the Treatment of Bacterial Vaginosis and Vulvovaginal Candidiasis: A Randomized, Single-blind, Phase 2, Controlled Trial
Abstract
Background: Bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC) present serious reproductive health risks and management challenges, with poor control attributed to survival of treatment-resistant biofilm communities. Boric acid is used in various regimens for non-albicans VVC and recurrent BV. We investigated safety and efficacy of a novel boric acid-based vaginal anti-infective with enhanced antibiofilm activity (TOL-463) in treating BV and VVC.
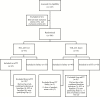
Methods: In this phase 2 randomized, investigator-blinded trial conducted at 2 sexual health clinics, women with BV or VVC were randomly assigned (1:1) to 7 nights of TOL-463 vaginal gel or insert. The primary test of cure (TOC) was clinical cure at day 9-12; safety was assessed at TOC and day 21-30.
Results: One hundred six participants (53 with BV, 36 VVC, 17 both) were enrolled; most were African American (69%). Clinical cure rate of BV at TOC was 59% (95% confidence interval [CI], 41%-75%) for TOL-463 insert and 50% (95% CI, 31%-69%) for TOL-463 gel, and for VVC, 92% (95% CI, 67%-99%) for TOL-463 insert and 81% (95% CI, 57%-93%) for TOL-463 gel. Both products were safe and well tolerated with no secondary cases of VVC; vulvovaginal burning was the most common adverse event (9.6%).
Conclusions: TOL-463, especially in vaginal insert form, is effective and safe in treating BV and VVC. Future studies should assess the potential role of TOL-463 as a biofilm disrupter in enhancing likelihood of cure relative to approved therapies, reducing recurrence rates, and combined with traditional antimicrobials.
Clinical trials registration: NCT02866227.
Keywords: TOL-463; bacterial vaginosis; boric acid; vaginal biofilm; vulvovaginal candidiasis.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Vaginal colonisation by probiotic lactobacilli and clinical outcome in women conventionally treated for bacterial vaginosis and yeast infection.BMC Infect Dis. 2015 Jul 3;15:255. doi: 10.1186/s12879-015-0971-3. BMC Infect Dis. 2015. PMID: 26137971 Free PMC article. Clinical Trial.
-
Prevalence of Candida glabrata and its response to boric acid vaginal suppositories in comparison with oral fluconazole in patients with diabetes and vulvovaginal candidiasis.Diabetes Care. 2007 Feb;30(2):312-7. doi: 10.2337/dc06-1469. Diabetes Care. 2007. PMID: 17259500 Clinical Trial.
-
Bacterial vaginosis, vulvovaginal candidiasis and trichomonal vaginitis among reproductive-aged women seeking primary healthcare in Sana'a city, Yemen.BMC Infect Dis. 2019 Oct 22;19(1):879. doi: 10.1186/s12879-019-4549-3. BMC Infect Dis. 2019. PMID: 31640583 Free PMC article.
-
Biofilms: An Underappreciated Mechanism of Treatment Failure and Recurrence in Vaginal Infections.Clin Infect Dis. 2015 Aug 15;61(4):601-6. doi: 10.1093/cid/civ353. Epub 2015 May 1. Clin Infect Dis. 2015. PMID: 25935553 Free PMC article. Review.
-
Boric acid for recurrent vulvovaginal candidiasis: the clinical evidence.J Womens Health (Larchmt). 2011 Aug;20(8):1245-55. doi: 10.1089/jwh.2010.2708. Epub 2011 Jul 20. J Womens Health (Larchmt). 2011. PMID: 21774671 Review.
Cited by
-
Assessing the Cervicovaginal Microbiota in the Context of hrHPV Infections: Temporal Dynamics and Therapeutic Strategies.mBio. 2022 Oct 26;13(5):e0161922. doi: 10.1128/mbio.01619-22. Epub 2022 Aug 18. mBio. 2022. PMID: 35980030 Free PMC article. Review.
-
Bacterial vaginosis.Nat Rev Dis Primers. 2025 Jun 19;11(1):43. doi: 10.1038/s41572-025-00626-1. Nat Rev Dis Primers. 2025. PMID: 40537474 Review.
-
Bacterial Vaginosis and Post-Operative Pelvic Infections.Healthcare (Basel). 2023 Apr 25;11(9):1218. doi: 10.3390/healthcare11091218. Healthcare (Basel). 2023. PMID: 37174760 Free PMC article. Review.
-
Protection and Risk: Male and Female Genital Microbiota and Sexually Transmitted Infections.J Infect Dis. 2021 Jun 16;223(12 Suppl 2):S222-S235. doi: 10.1093/infdis/jiaa762. J Infect Dis. 2021. PMID: 33576776 Free PMC article. Review.
-
The right bug in the right place: opportunities for bacterial vaginosis treatment.NPJ Biofilms Microbiomes. 2022 May 2;8(1):34. doi: 10.1038/s41522-022-00295-y. NPJ Biofilms Microbiomes. 2022. PMID: 35501321 Free PMC article. Review.
References
-
- Diflucan [package insert]. New York, NY: Pfizer; 2018.
-
- Nuvessa [package insert]. Irvine, CA: Allergan; 2016.
-
- Aguin TJ, Sobel JD. Vulvovaginal candidiasis in pregnancy. Curr Infect Dis Rep 2015; 17:462. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

