Safety and Immunogenicity of Pneumococcal Conjugate Vaccines in a High-risk Population: A Randomized Controlled Trial of 10-Valent and 13-Valent Pneumococcal Conjugate Vaccine in Papua New Guinean Infants
- PMID: 30184183
- PMCID: PMC6481999
- DOI: 10.1093/cid/ciy743
Safety and Immunogenicity of Pneumococcal Conjugate Vaccines in a High-risk Population: A Randomized Controlled Trial of 10-Valent and 13-Valent Pneumococcal Conjugate Vaccine in Papua New Guinean Infants
Abstract
Background: There are little data on the immunogenicity of PCV10 and PCV13 in the same high-risk population.
Methods: PCV10 and PCV13 were studied head-to-head in a randomized controlled trial in Papua New Guinea in which 262 infants received 3 doses of PCV10 or PCV13 at 1, 2, and 3 months of age. Serotype-specific immunoglobulin G (IgG) concentrations, and pneumococcal and nontypeable Haemophilus influenzae (NTHi) carriage were assessed prevaccination and at 4 and 9 months of age. Infants were followed up for safety until 9 months of age.
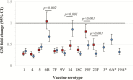
Results: One month after the third dose of PCV10 or PCV13, ˃80% of infants had IgG concentrations ≥0.35µg/mL for vaccine serotypes, and 6 months postvaccination IgG concentrations ≥0.35 µg/mL were maintained for 8/10 shared PCV serotypes in > 75% of children vaccinated with either PCV10 or PCV13. Children carried a total of 65 different pneumococcal serotypes (plus nonserotypeable). At 4 months of age, 92% (95% confidence interval [CI] 85-96) of children vaccinated with PCV10 and 81% (95% CI 72-88) vaccinated with PCV13 were pneumococcal carriers (P = .023), whereas no differences were seen at 9 months of age, or for NTHi carriage. Both vaccines were well tolerated and not associated with serious adverse events.
Conclusions: Infant vaccination with 3 doses of PCV10 or PCV13 is safe and immunogenic in a highly endemic setting; however, to significantly reduce pneumococcal disease in these settings, PCVs with broader serotype coverage and potency to reduce pneumococcal carriage are needed.
Clinical trials registration: NCT01619462.
Keywords: S. pneumonia; Papua New Guinea; antibodies; carriage; pneumococcal conjugate vaccine.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
-
- World Health Organization. Immunization coverage. Fact sheet Available at: http://www.who.int/mediacentre/factsheets/fs378/en/. Accessed 31 August 2017.
-
- The Boston Consulting Group, The Vaccine Alliance. The advance market commitment pilot for pneumococcal vaccines: outcomes and impact evaluation, 2015. Available at: http://www.gavi.org/results/evaluations/pneumococcal-amc-outcomes-and-im.... Accessed 12 December 2017.
-
- Prymula R, Peeters P, Chrobok V, et al. . Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006; 367:740–8. - PubMed
-
- Mudhune S, Wamae M; Network Surveillance for Pneumococcal Disease in the East African Region Report on invasive disease and meningitis due to Haemophilus influenzae and Streptococcus pneumonia from the Network for Surveillance of Pneumococcal Disease in the East African Region. Clin Infect Dis 2009; 48 Suppl 2:S147–52. - PMC - PubMed
-
- Van Eldere J, Slack MP, Ladhani S, Cripps AW. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis 2014; 14:1281–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

