PROCOMIDA, a Food-Assisted Maternal and Child Health and Nutrition Program, Reduces Child Stunting in Guatemala: A Cluster-Randomized Controlled Intervention Trial
- PMID: 30184223
- PMCID: PMC6118165
- DOI: 10.1093/jn/nxy138
PROCOMIDA, a Food-Assisted Maternal and Child Health and Nutrition Program, Reduces Child Stunting in Guatemala: A Cluster-Randomized Controlled Intervention Trial
Abstract
Background: Food-assisted maternal and child health and nutrition (FA-MCHN) programs may foster child growth during the first 1000 d (pregnancy and the first 2 y of a child's life), but evidence is scant.
Objective: We evaluated the impact of an FA-MCHN program, PROCOMIDA, on linear growth (stunting [length-for-age z score (LAZ) < -2] and length-for-age difference [LAD]) among children aged 1-24 mo. PROCOMIDA was implemented in Guatemala by Mercy Corps and was available to beneficiaries throughout the first 1000 d.
Methods: We used a longitudinal, cluster-randomized controlled trial with groups varying in family ration sizes [full (FFR), reduced (RFR), and none (NFR)] and individual ration types provided to mothers (pregnancy to 6 mo postpartum) and children (6-24 mo of age) [corn-soy blend (CSB), lipid-based nutrient supplement (LNS), micronutrient powder (MNP)]: 1) FFR + CSB (n = 576); 2) RFR + CSB (n = 575); 3) NFR + CSB (n = 542); 4) FFR + LNS (n = 550); 5) FFR + MNP (n = 587); 6) control (n = 574). Program impacts compared with control, and differential impacts between groups varying family ration size or individual ration type, were assessed through the use of linear mixed-effects models and post hoc simple effect tests (significant if P < 0.05).
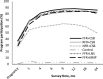
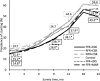
Results: PROCOMIDA significantly reduced stunting at age 1 mo in FFR + CSB, RFR + CSB, and FFR + MNP groups compared with control [5.05, 4.06, and 3.82 percentage points (pp), respectively]. Stunting impact increased by age 24 mo in FFR + CSB and FFR + MNP relative to control (impact = 11.1 and 6.5 pp at age 24 mo, respectively). For CSB recipients, the FFR compared with RFR or NFR significantly reduced stunting (6.47-9.68 pp). CSB reduced stunting significantly more than LNS at age 24 mo (8.12 pp).
Conclusions: FA-MCHN programs can reduce stunting during the first 1000 d, even in relatively energy/food-secure populations. Large family rations with individual rations of CSB or MNP were most effective. The widening of impact as children age highlights the importance of intervening throughout the full first 1000 d. This trial was registered at clinicaltrials.gov as NCT01072279.
Figures

References
-
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R et al. . Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. - PubMed
-
- International Food Policy Research Institute (IFPRI) Global nutrition report 2016: from promise to impact: ending malnutrition by 2030. Washington (DC): International Food Policy Research Institute; 2016.
-
- Delgado HL. Technical report. Status and trends in chronic malnutrition in Guatemala. Chevy Chase, MD: University Research Co., LLC; 2010.
-
- Schroeder DG, Martorell R, Rivera JA, Ruel MT, Habicht JP. Age differences in the impact of nutritional supplementation on growth. J Nutr 1995;125:1051S–9S. - PubMed
-
- Martorell R, Schroeder DG, Rivera JA, Kaplowitz HJ. Patterns of linear growth in rural Guatemalan adolescents and children. J Nutr 1995;125:1060S–7S. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

