Proteinuria and cholesterol reduction are independently associated with less renal function decline in statin-treated patients; a post hoc analysis of the PLANET trials
- PMID: 30184238
- PMCID: PMC6775475
- DOI: 10.1093/ndt/gfy159
Proteinuria and cholesterol reduction are independently associated with less renal function decline in statin-treated patients; a post hoc analysis of the PLANET trials
Abstract
Background: Statins have shown multiple effects on different renal risk factors such as lowering of total cholesterol (TC) and lowering of urine protein:creatinine ratio (UPCR). We assessed whether these effects of statins vary between individuals, the extent of discordance of treatment effects on both TC and UPCR within an individual, and the association of responses in TC and UPCR with estimated glomerular filtration rate (eGFR) decline.
Methods: The PLANET I and II (Renal effects of Rosuvastatin and Atorvastatin in Patients Who Have Progressive Renal Disease) trials examined effects of atorvastatin and rosuvastatin on proteinuria and renal function in patients with proteinuria. We post hoc analysed 471 therapy-adherent proteinuric patients from the two trials and assessed the individual variability in UPCR and TC response from 0 to 14 weeks and whether these responses were predictive of eGFR decline during the subsequent 9 months of follow-up.
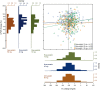
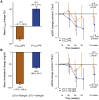
Results: UPCR and TC response varied between individuals: mean UPCR response was -1.3% (5th-95th percentile -59.9 to 141.8) and mean TC response was -93.9 mg/dL (-169.1 to -26.9). Out of 471 patients, 123 (26.1%) showed a response in UPCR but not in TC, and 96 (20.4%) showed a response in TC but not in UPCR. eGFR (mL/min/1.73 m2) did not decrease significantly from baseline in both UPCR responders [0.4; 95% confidence interval (CI) -1.6 to 0.9; P = 0.54] and TC responders (0.3; 95% CI -1.8 to 1.1; P = 0.64), whereas UPCR and TC non-responders showed a significant decline in eGFR from baseline (1.8; 95% CI 0.6-3.0; P = 0.004 and 1.7; 95% CI 0.5-2.9; P = 0.007, respectively). A lack of response in both parameters resulted in the fastest rate of eGFR decline (2.1; 95% CI 0.5-3.7; P = 0.01). These findings were not different for rosuvastatin or atorvastatin.
Conclusions: Statin-induced changes in cholesterol and proteinuria vary between individuals and do not run in parallel within an individual. The initial fall in cholesterol and proteinuria is independently associated with a reduction in eGFR decline. This highlights the importance of monitoring both cholesterol and proteinuria after initiating statin therapy.
Keywords: cholesterol; proteinuria; renal function; response variability; statins.
© The Author(s) 2018. Published by Oxford University Press on behalf of ERA-EDTA.
Figures

References
-
- Palmer SC, Navaneethan SD, Craig JC. et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev 2014; CD007784; doi: 10.1002/1465?>1858.CD007784.pub2. - PubMed
-
- de Zeeuw D, Anzalone DA, Cain VA. et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. Lancet Diabetes Endocrinol 2015; 3: 181–190 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

