Molecular Insights into Human Hereditary Apolipoprotein A-I Amyloidosis Caused by the Glu34Lys Mutation
- PMID: 30184436
- PMCID: PMC11259198
- DOI: 10.1021/acs.biochem.8b00817
Molecular Insights into Human Hereditary Apolipoprotein A-I Amyloidosis Caused by the Glu34Lys Mutation
Abstract
Hereditary apolipoprotein A-I (apoA-I) amyloidosis is a life-threatening incurable genetic disorder whose molecular underpinnings are unclear. In this disease, variant apoA-I, the major structural and functional protein of high-density lipoprotein, is released in a free form, undergoes an α-helix to intermolecular cross-β-sheet conversion along with a proteolytic cleavage, and is deposited as amyloid fibrils in various organs, which can cause organ damage and death. Glu34Lys is the only known charge inversion mutation in apoA-I that causes human amyloidosis. To elucidate the structural underpinnings of the amyloidogenic behavior of Glu34Lys apoA-I, we generated its recombinant globular N-terminal domain (residues 1-184) and compared the conformation and dynamics of its lipid-free form with those of two other naturally occurring apoA-I variants, Phe71Tyr (amyloidogenic) and Leu159Arg (non-amyloidogenic). All variants showed reduced structural stability and altered aromatic residue packing. The greatest decrease in stability was observed in the non-amyloidogenic variant, suggesting that amyloid formation is driven by local structural perturbations at sensitive sites. Molecular dynamics simulations revealed local helical unfolding and suggested that transient opening of the Trp72 side chain induced mutation-dependent structural perturbations in a sensitive region, including the major amyloid hot spot residues Leu14-Leu22. We posit that a shift from the "closed" to the "open" orientation of the Trp72 side chain modulates structural protection of amyloid hot spots, suggesting a previously unknown early step in the protein misfolding pathway.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

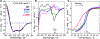
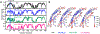

References
-
- Nichols WC, Dwulet FE, Liepnieks J, and Benson MD (1988) Variant apolipoprotein AI as a major constituent of a human hereditary amyloid. Biochem. Biophys. Res. Commun. 156, 762–768. - PubMed
-
- Rosenson RS, Brewer HB Jr., Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, and Yvan-Charvet L (2012) Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 125, 1905–1919. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

