Aliphatic C-H Oxidations for Late-Stage Functionalization
- PMID: 30185033
- PMCID: PMC6410715
- DOI: 10.1021/jacs.8b05195
Aliphatic C-H Oxidations for Late-Stage Functionalization
Abstract
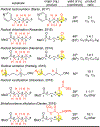
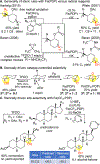
The atomistic change of C( sp3)-H to C( sp3)-O can have a profound impact on the physical and biological properties of small molecules. Traditionally, chemical synthesis has relied on pre-existing functionality to install new functionality, and directed approaches to C-H oxidation are an extension of this logic. The impact of developing undirected C-H oxidation reactions with controlled site-selectivity is that scientists gain the ability to diversify complex structures at sites remote from existing functionality, without having to carry out individual de novo syntheses. This Perspective offers a historical view of why, as recently as 2007, it was thought that the differences between aliphatic C-H bonds of the same bond type (for example, 2° aliphatic) were not large enough to distinguish them preparatively with small-molecule catalysis in the absence of directing groups or molecular recognition elements. We give an account of the discovery of Fe(PDP)-catalyzed non-directed aliphatic C-H hydroxylations and how the electronic, steric, and stereoelectronic rules for predicting site-selectivity that emerged have affected a shift in how the chemical community views the reactivity among these bonds. The discovery that site-selectivity could be altered by tuning the catalyst [i.e., Fe(CF3-PDP)] with no changes to the substrate or reaction now gives scientists the ability to exert control on the site of oxidation on a range of functionally and topologically diverse compounds. Collectively, these findings have made possible the emerging area of late-stage C-H functionalizations for streamlining synthesis and derivatizing complex molecules.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


















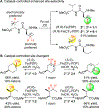






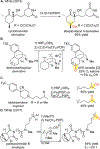
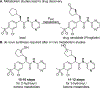


References
-
- Bergman RG Nature 2007, 446, 391. - PubMed
-
- Godula K; Sames D Science 2006, 312, 67. - PubMed
-
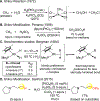
- (a) The hydroxyl group of Lyral® imparts the Lilly of the Vally odor to this aroma chemical and removal of the hydroxyl leaves the compound odorless: Rossiter KJ Chem. Rev 1996, 96, 3201. - PubMed
- (b) The natural product (+)-valencene that gives oranges their characteristic flavor can be enzymatically oxidized at just one site to furnish b-nootkatone, a compound that now tastes like grapefruit: Rouseff R; Perez-Cacho PR Citrus Flavour. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger RG, Eds.; Springer-Verlag: Berlin, Heidelberg, 2007; pp 117.
-
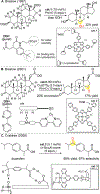
- (a) Paclitaxel (Taxol®) a highly oxygenated diterpenoid clinically used for the treatment of a range of aggressive cancers (ovarian, breast, lung, pancreatic) looses its ability to polymerize tubulin and to effect cytoxicity upon removal of the C2 benzoate: Chen S-H; Farina V Paclitaxel Structure-Activity Relationships and Core Skeletal Rearrangements. In Taxane Anticancer Agents: Basic Science and Current Status; Georg GI; Chen TT; Ojima I; Vyas DM, Eds.; ACS Symposium Series 583; American Chemical Society: Washington, DC, 1994; pp 247.
- (b) Erythromycin A, a clinically used antibiotic, is rendered 2–4 fold less active upon removal of the C6 hydroxyl moiety: Weber JW; Leung JO; Swanson SJ; Idler KB; McAlpine JB Science 1991, 252, 114. - PubMed
- (c) Zambias RA; Hammond ML; Heck JV; Bartizal K; Trainor C; Abruzzo G; Schmatz DM; Nollstadt KM J. Med. Chem 1992, 35, 2843. - PubMed
- (d) The hydroxyl functionality found in HIV (human immunodeficiency virus) protease inhibitors are critical for binding to the active site via H-bonding to catalytic aspartate residues. Removal of the hydroxyl group in the HIV medication ritonavir has led to the drug cobicistat, having greatly reduced anti-HIV activity but retaining potent inhibitory activity of liver enzymes (CYP3A): Xu L; Liu H; Hong A; Vivian R; Murray BP; Callebaut C; Choi YC; Lee MS; Chau J; Tsai LK; Stray KM; Strickley RG; Wang J; Tong L; Swaminathan S; Rhodes GR; Desai MC Bioorg. Med. Chem. Lett 2014, 24, 995. - PubMed
-
- Davies NM Clin. Pharmacokinet 1998, 34, 101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

