Protocol for a Delphi consensus exercise to identify a core set of criteria for selecting health related outcome measures (HROM) to be used in primary health care
- PMID: 30185172
- PMCID: PMC6123958
- DOI: 10.1186/s12875-018-0831-5
Protocol for a Delphi consensus exercise to identify a core set of criteria for selecting health related outcome measures (HROM) to be used in primary health care
Abstract
Background: Promoting the collection and use of health related outcome measures (HROM) in daily practice has long been a goal for improving and assessing the effectiveness of care provided to patients. However, there has been a lack of consensus on what criteria to use to select outcomes or instruments, particularly in the context of primary health care settings where patients present with multiple concurrent health conditions and interventions are whole-health and person-focused. The purpose of this proposed study is to undertake a formal consensus exercise to establish criteria for selecting HROM (including patient-reported (PRO or PROM), observer-reported (ObsR)), clinician-reported (ClinRO) and performance related outcomes (PerfO) for use in shared decision-making, or in assessing, screening or monitoring health status in primary health care settings.
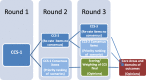
Methods: A Delphi consensus online survey will be developed. Criteria for the Delphi panel participants to consider were selected from a targeted literature search. These initial criteria (n = 35) were grouped into four categories within which items will be presented in the Delphi survey, with the option to suggest additional items. Panel members invited to participate will include primary health care practitioners and administrators, policy-makers, researchers, and experts in HROM development; patients will be excluded. Standard Delphi methodology will be employed with an expectation of at least 3 rounds to achieve consensus (75% agreement). As the final list of criteria for selecting HROM emerges, panel members will be asked to provide opinions about potential weighting of items. The Delphi survey was approved by the Ethics Committee in the Faculty of Health Sciences at McMaster University.
Discussion: Previous literature establishing criteria for selecting HROM were developed with a focus on patient reported outcomes, psychological/ behavioural outcomes or outcomes for minimum core outcome sets in clinical trials. Although helpful, these criteria may not be applicable and feasible for application in a primary health care context where patients with multi-morbidity and complex interventions are typical and the constraints of providing health services differ from those in research studies. The findings from this Delphi consensus study will address a gap for establishing consensus on criteria for selecting HROM for use across primary health care settings.
Keywords: Delphi consensus; Primary health care; Protocol; Selection of outcomes.
Conflict of interest statement
Ethics approval and consent to participate
Approval for this Delphi study was obtained from the Research Ethics Committee (RB 15–207) at McMaster University, Ontario, Canada. Delphi participants will be required to consent to participation in the online survey.
Competing interests
Research.
Parminder Raina holds a Tier 1 Canada Research Chair in Geroscience and the Raymond and Margaret Labarge Chair in Research and Knowledge Application for Optimal Aging
Lauren Griffith PhD is supported by a Canadian Institutes of Health Research New Investigators Award and the McLaughlin Foundation Professorship in Population and Public Health
The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Reeve BB, Wyrwich KW, Wu AW, Velikova G, Terwee CB, Snyder CF, Schwartz C, Revicki DA, Moinpour CM, McLeod LD, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22(8):1889–1905. doi: 10.1007/s11136-012-0344-y. - DOI - PubMed
-
- Estabrooks PA, Boyle M, Emmons KM, Glasgow RE, Hesse BW, Kaplan RM, Krist AH, Moser RP, Taylor MV. Harmonized patient-reported data elements in the electronic health record: supporting meaningful use by primary care action on health behaviors and key psychosocial factors. J Am Med Inform Assoc. 2012;19(4):575–582. doi: 10.1136/amiajnl-2011-000576. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

