Lantern-shaped screw loaded with autologous bone for treating osteonecrosis of the femoral head
- PMID: 30185196
- PMCID: PMC6123930
- DOI: 10.1186/s12891-018-2243-z
Lantern-shaped screw loaded with autologous bone for treating osteonecrosis of the femoral head
Abstract
Background: Treatment for osteonecrosis of the femoral head (ONFH) in young individuals remains controversial. We developed a lantern-shaped screw, which was designed to provide mechanical support for the femoral head to prevent its collapse, for the treatment of ONFH. The purpose of this study was to investigate the efficacy and safety of the lantern-shaped screw loaded with autologous bone for the treatment of pre-collapse stages of ONFH.
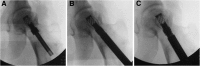
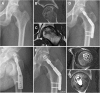
Methods: Thirty-two patients were randomly divided into two groups: the lantern-shaped screw group (core decompression and lantern-shaped screw loaded with autogenous bone) and the control group (core decompression and autogenous bone graft). During 36 months follow-up after surgery, treatment results in patients were assessed by X-ray and computed tomography (CT) scanning as well as functional recovery Harris hip score (HHS).
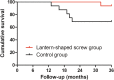
Results: Successful clinical results were achieved in 15 of 16 hips (94%) in the lantern-shaped screw group compared with 10 of 16 hips (63%) in the control group (p = 0.0325). Successful radiological results were achieved in 14 of 16 hips (88%) in the lantern-shaped screw group compared with 8 of 16 hips (50%) in the control group (P = 0.0221).
Conclusion: The lantern-shaped screw loaded with autologous bone for the treatment of pre-collapse stages of ONFH is effective and results in preventing progression of ONFH and reducing the risk of femoral head collapse.
Trial registration: The trial registration number: ChiCTR-TRC-13004078 (retrospectively registered at 2013-11-28).
Keywords: Autogenous bone graft; Core decompression; Lantern-shaped screw; Osteonecrosis of the femoral head.
Conflict of interest statement
Ethics approval and consent to participate
This prospective and randomized controlled study was registered in the Chinese Clinical Trial Registry (ChiCTR-TRC-13004078). This study was carried out in accordance with the guidelines of the Declaration of Helsinki. All experimental protocols were approved by our institutional review board (Xiamen University Ethical Review Committee), and written informed consent was obtained from all study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

