The putative tumour suppressor miR-1-3p modulates prostate cancer cell aggressiveness by repressing E2F5 and PFTK1
- PMID: 30185212
- PMCID: PMC6125869
- DOI: 10.1186/s13046-018-0895-z
The putative tumour suppressor miR-1-3p modulates prostate cancer cell aggressiveness by repressing E2F5 and PFTK1
Abstract
Background: Previous studies report that miR-1-3p, a member of the microRNA-1 family (miR-1), and functions as a tumor suppressor in several different cancers. However, little is known regarding the biological role and intrinsic regulatory mechanisms of miR-1-3p in prostate cancer (PCa).
Methods: In this study, the expression levels of miR-1-3p were first examined in PCa cell lines and tumor tissues by RT-qPCR and bioinformatics. The in vitro and in vivo functional effect of miR-1-3p was examined further. A luciferase reporter assay was conducted to confirm target associations.
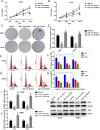
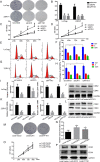
Results: We found that miR-1-3p was significantly downregulated in advanced PCa tissues and cell lines. Low miR-1-3p levels were strongly associated with aggressive clinicopathological features and poor prognosis in PCa patients. Ectopic expression of miR-1-3p in 22RV1 and LncaP cells was sufficient to prevent tumor cell growth and cell cycle progression in vitro and in vivo. Further mechanistic studies revealed that miR-1-3p could directly target the mRNA 3'- untranslated region (3'- UTR) of two central cell cycle genes, E2F5 and PFTK1, and could suppress their mRNA and protein expression. In addition, knockdown of E2F5 and PFTK1 mimicked the tumor-suppressive effects of miR-1-3p overexpression on PCa progression. Conversely, concomitant knockdown of miR-1-3p and E2F5 and PFTK1 substantially reversed the inhibitory effects of either E2F5 or PFTK1 silencing alone.
Conclusion: These data highlight an important role for miR-1-3p in the regulation of proliferation and cell cycle in the molecular etiology of PCa and indicate the potential for miR-1-3p in applications furthering PCa prognostics and therapeutics.
Keywords: Proliferation; Prostate cancer; Target gene; microRNA.
Conflict of interest statement
Ethics approval and consent to participate
All protocols were approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and informed consent was obtained from all patients before surgery.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

