Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models
- PMID: 30185216
- PMCID: PMC6125882
- DOI: 10.1186/s13058-018-1037-4
Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models
Abstract
Background: Breast cancer has been considered not highly immunogenic, and few patients benefit from current immunotherapies. However, new strategies are aimed at changing this paradigm. In the present study, we examined the in vivo activity of a humanized anti-programmed cell death protein 1 (anti-PD-1) antibody against triple-negative breast cancer (TNBC) patient-derived xenograft (PDX) tumor models.
Methods: To circumvent some of the limitations posed by the lack of appropriate animal models in preclinical studies of immunotherapies, partially human leukocyte antigen-matched TNBC PDX tumor lines from our collection, as well as human melanoma cell lines, were engrafted in humanized nonobese diabetic/severe combined immunodeficiency IL2Rγnull (hNSG) mice obtained by intravenous injection of CD34+ hematopoietic stem cells into nonlethally irradiated 3-4-week-old mice. After both PDXs and melanoma cell xenografts reached ~ 150-200 mm3, animals were treated with humanized anti-PD-1 antibody or anti-CTLA-4 and evaluated for tumor growth, survival, and potential mechanism of action.
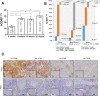
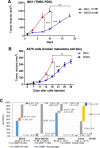
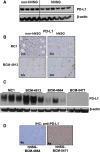
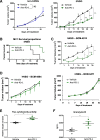
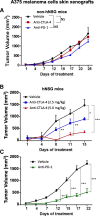
Results: Human CD45+, CD20+, CD3+, CD8+, CD56+, CD68+, and CD33+ cells were readily identified in blood, spleen, and bone marrow collected from hNSG, as well as human cytokines in blood and engrafted tumors. Engraftment of TNBC PDXs in hNSG was high (~ 85%), although they grew at a slightly slower pace and conserved their ability to generate lung metastasis. Human CD45+ cells were detectable in hNSG-harbored PDXs, and consistent with clinical observations, anti-PD-1 antibody therapy resulted in both a significant reduction in tumor growth and increased survival in some of the hNSG PDX tumor lines, whereas no such effects were observed in the corresponding non-hNSG models.
Conclusions: This study provides evidence associated with anti-PD-1 immunotherapy against TNBC tumors supporting the use of TNBC PDXs in humanized mice as a model to overcome some of the technical difficulties associated with the preclinical investigation of immune-based therapies.
Keywords: Anti-PD-1; Humanized mouse model; Immunotherapy; PD-L1; TNBC; Triple-negative breast cancer.
Conflict of interest statement
Ethics approval and consent to participate
Tissues used to generate the patient-derived xenografts were collected from consenting patients following institutional review board-approved protocols at clinics in the Baylor College of Medicine and Ben Taub General Hospital (Houston, TX, USA), as previously reported [12], and at Houston Methodist Hospital Cancer Center (protocol Pro00005346).
Consent for publication
Not applicable, because the present article does not contain any individual person’s data in any form.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts.J Immunother Cancer. 2019 Feb 8;7(1):37. doi: 10.1186/s40425-019-0518-z. J Immunother Cancer. 2019. PMID: 30736857 Free PMC article.
-
Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy.FASEB J. 2018 Mar;32(3):1537-1549. doi: 10.1096/fj.201700740R. Epub 2018 Jan 3. FASEB J. 2018. PMID: 29146734 Free PMC article.
-
Establishment of peripheral blood mononuclear cell-derived humanized lung cancer mouse models for studying efficacy of PD-L1/PD-1 targeted immunotherapy.MAbs. 2018 Nov-Dec;10(8):1301-1311. doi: 10.1080/19420862.2018.1518948. Epub 2018 Oct 2. MAbs. 2018. PMID: 30204048 Free PMC article.
-
Immunotherapy in triple-negative breast cancer.Med Oncol. 2017 Dec 18;35(1):13. doi: 10.1007/s12032-017-1071-6. Med Oncol. 2017. PMID: 29255938 Review.
-
Triple negative breast cancer: Key role of Tumor-Associated Macrophages in regulating the activity of anti-PD-1/PD-L1 agents.Biochim Biophys Acta Rev Cancer. 2018 Jan;1869(1):78-84. doi: 10.1016/j.bbcan.2017.10.007. Epub 2017 Nov 7. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29126881 Review.
Cited by
-
'Omics Approaches to Explore the Breast Cancer Landscape.Front Cell Dev Biol. 2020 Jan 22;7:395. doi: 10.3389/fcell.2019.00395. eCollection 2019. Front Cell Dev Biol. 2020. PMID: 32039208 Free PMC article. Review.
-
Characterization of human cancer xenografts in humanized mice.J Immunother Cancer. 2020 Mar;8(1):e000416. doi: 10.1136/jitc-2019-000416. J Immunother Cancer. 2020. PMID: 32217760 Free PMC article.
-
The Essential Factors of Establishing Patient-derived Tumor Model.J Cancer. 2021 Jan 1;12(1):28-37. doi: 10.7150/jca.51749. eCollection 2021. J Cancer. 2021. PMID: 33391400 Free PMC article. Review.
-
Efficacy and safety profiles of programmed cell death-1/programmed cell death ligand-1 inhibitors in the treatment of triple-negative breast cancer: A comprehensive systematic review.Oncol Rev. 2019 Oct 10;13(2):425. doi: 10.4081/oncol.2019.425. eCollection 2019 Jul 22. Oncol Rev. 2019. PMID: 31857857 Free PMC article.
-
The Application Progress of Patient-Derived Tumor Xenograft Models After Cholangiocarcinoma Surgeries.Front Oncol. 2021 Jul 22;11:628636. doi: 10.3389/fonc.2021.628636. eCollection 2021. Front Oncol. 2021. PMID: 34367944 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

