A phase Ib study of pictilisib (GDC-0941) in combination with paclitaxel, with and without bevacizumab or trastuzumab, and with letrozole in advanced breast cancer
- PMID: 30185228
- PMCID: PMC6125885
- DOI: 10.1186/s13058-018-1015-x
A phase Ib study of pictilisib (GDC-0941) in combination with paclitaxel, with and without bevacizumab or trastuzumab, and with letrozole in advanced breast cancer
Abstract
Background: This phase Ib study (NCT00960960) evaluated pictilisib (GDC-0941; pan-phosphatidylinositol 3-kinase inhibitor) plus paclitaxel, with and without bevacizumab or trastuzumab, or in combination with letrozole, in patients with locally recurrent or metastatic breast cancer.
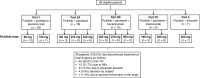
Methods: This was a three-part multischedule study. Patients in parts 1 and 2, which comprised 3 + 3 dose escalation and cohort expansion stages, received pictilisib (60-330 mg) plus paclitaxel (90 mg/m2) with and without bevacizumab (10 mg/kg) or trastuzumab (2-4 mg/kg). In part 3, patients received pictilisib (260 mg) plus letrozole (2.5 mg). Primary objectives were evaluation of safety and tolerability, identification of dose-limiting toxicities (DLTs) and the maximum tolerated dose (MTD) of pictilisib, and recommendation of a phase II dosing regimen. Secondary endpoints included pharmacokinetics and preliminary antitumor activity.
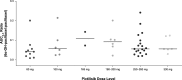
Results: Sixty-nine patients were enrolled; all experienced at least one adverse event (AE). Grade ≥ 3 AEs, serious AEs, and AEs leading to death were reported in 50 (72.5%), 21 (30.4%), and 2 (2.9%) patients, respectively. Six (8.7%) patients reported a DLT, and the MTD and recommended phase II pictilisib doses were established where possible. There was no pictilisib-paclitaxel drug-drug interaction. Two (3.4%) patients experienced complete responses, and 17 (29.3%) patients had partial responses.
Conclusions: Combining pictilisib with paclitaxel, with and without bevacizumab or trastuzumab, or letrozole, had a manageable safety profile in patients with locally recurrent or metastatic breast cancer. The combination had antitumor activity, and the additive effect of pictilisib supported further investigation in a randomized study.
Trial registration: ClinicalTrials.gov, NCT00960960 . Registered on August 13, 2009.
Keywords: GDC-0941; PI3K; Pictilisib.
Conflict of interest statement
Ethics approval and consent to participate
This study was conducted in accordance with good clinical practice guidelines and the Declaration of Helsinki. Approval of the protocol and any accompanying material provided to the patients was obtained from independent ethics committees at participating institutions (Table 4). All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
PS has received travel support from Roche for presentation of parts of this work at the 37th San Antonio Breast Cancer Symposium in 2014, institutional research support from Genentech for exploring pictilisib in patient-derived xenograft models of sarcoma, and institutional technical support from Roche (donation of a LightCycler). In addition, and outside the submitted work, PS has received honoraria (institutional support) from Daiichi Sankyo, Eisai, Eli Lilly, Medpace, Novartis, and Swedish Orphan Biovitrium; consulting or advisory role institutional support from Sixth Element Capital, Adaptimmune, Amcure, AstraZeneca, Bayer, Blueprint Medicines, BMS, Boehringer Ingelheim, Cristal Therapeutics, Daiichi Sankyo, Eisai, Eli Lilly, Epizyme, Genzyme, Ipsen, Loxo Oncology, Medpace, Nektar, Novartis, Philogen, PIQUR Therapeutics, and Plexxikon; speaker’s bureau institutional support from Bayer, Eisai, Eli Lilly, GSK, Novartis, PharmaMar, and Swedish Orphan Biovitrium; research funding (institutional support) from Bayer, Blueprint Medicines, CoBioRes NV, Exelixis, GSK, Novartis, and Plexxikon; and institutional support for travel, accommodation, and expenses from Sixth Element Capital, Adaptimmune, Amcure, AstraZeneca, Bayer, Blueprint Medicines, BMS, Boehringer Ingelheim, Cristal Therapeutics, Daiichi Sankyo, Eisai, Eli Lilly, Epizyme, Genzyme, GSK, Ipsen, Loxo Oncology, Medpace, Nektar, Novartis, PharmaMar, Philogen, PIQUR Therapeutics, Plexxikon, and Swedish Orphan Biovitrium. IAM has received research support from Novartis and Pfizer and advisory board honoraria from Novartis. HW has received advisory board honoraria, speaker‘s fees, and travel support from Roche. SG, IR, KMM, JMS, VWN, and SMS are employees of Genentech/Roche. EW has received research support from Genentech, an advisory board honorarium from Genentech, and scientific advisory board support from Leap Pharmaceuticals. SC and SD declare that they have no potential conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
A phase IB dose-escalation study of the safety and pharmacokinetics of pictilisib in combination with either paclitaxel and carboplatin (with or without bevacizumab) or pemetrexed and cisplatin (with or without bevacizumab) in patients with advanced non-small cell lung cancer.Eur J Cancer. 2017 Nov;86:186-196. doi: 10.1016/j.ejca.2017.08.027. Epub 2017 Oct 6. Eur J Cancer. 2017. PMID: 28992562 Clinical Trial.
-
A Phase I Dose-Escalation Study of the Safety and Pharmacokinetics of Pictilisib in Combination with Erlotinib in Patients with Advanced Solid Tumors.Oncologist. 2017 Dec;22(12):1491-1499. doi: 10.1634/theoncologist.2017-0090. Epub 2017 Aug 10. Oncologist. 2017. PMID: 28798270 Free PMC article. Clinical Trial.
-
Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study.Ann Oncol. 2016 Nov;27(11):2059-2066. doi: 10.1093/annonc/mdw320. Epub 2016 Aug 29. Ann Oncol. 2016. PMID: 27573562 Clinical Trial.
-
Phase Ia/Ib study of the pan-class I PI3K inhibitor pictilisib (GDC-0941) administered as a single agent in Japanese patients with solid tumors and in combination in Japanese patients with non-squamous non-small cell lung cancer.Invest New Drugs. 2017 Feb;35(1):37-46. doi: 10.1007/s10637-016-0382-3. Epub 2016 Aug 27. Invest New Drugs. 2017. PMID: 27565810 Clinical Trial.
-
Phase Ib study of the MEK inhibitor cobimetinib (GDC-0973) in combination with the PI3K inhibitor pictilisib (GDC-0941) in patients with advanced solid tumors.Invest New Drugs. 2020 Apr;38(2):419-432. doi: 10.1007/s10637-019-00776-6. Epub 2019 Apr 24. Invest New Drugs. 2020. PMID: 31020608 Clinical Trial.
Cited by
-
PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects.Int J Mol Sci. 2021 Mar 27;22(7):3464. doi: 10.3390/ijms22073464. Int J Mol Sci. 2021. PMID: 33801659 Free PMC article. Review.
-
FOXO transcriptional activity is associated with response to chemoradiation in EAC.J Transl Med. 2022 Apr 25;20(1):183. doi: 10.1186/s12967-022-03376-w. J Transl Med. 2022. PMID: 35468793 Free PMC article.
-
A high-throughput drug combination screen of targeted small molecule inhibitors in cancer cell lines.Sci Data. 2019 Oct 29;6(1):237. doi: 10.1038/s41597-019-0255-7. Sci Data. 2019. PMID: 31664030 Free PMC article.
-
Targeting Breast Cancer: An Overlook on Current Strategies.Int J Mol Sci. 2023 Feb 11;24(4):3643. doi: 10.3390/ijms24043643. Int J Mol Sci. 2023. PMID: 36835056 Free PMC article. Review.
-
Role of Molecular Targeted Therapeutic Drugs in Treatment of Breast Cancer: A Review Article.Glob Med Genet. 2023 May 23;10(2):79-86. doi: 10.1055/s-0043-57247. eCollection 2023 Jun. Glob Med Genet. 2023. PMID: 37228871 Free PMC article. Review.
References
-
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer. Version 2. 2016. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 14 Oct 2016. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

