The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5
- PMID: 30185232
- PMCID: PMC6125951
- DOI: 10.1186/s13045-018-0656-7
The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5
Abstract
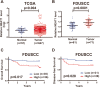
Background: The long noncoding RNA nuclear-enriched abundant transcript 1 (NEAT1) has been reported to be overexpressed in colorectal cancer (CRC). However, its underlying mechanisms in the progression of CRC have not been well studied.
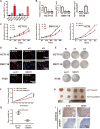
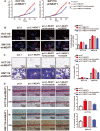
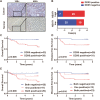
Methods: To investigate the clinical significance of NEAT1, we analyzed its expression levels in a publicly available dataset and in 71 CRC samples from Fudan University Shanghai Cancer Center. Functional assays, including the CCK8, EdU, colony formation, wound healing, and Transwell assays, were used to determine the oncogenic role of NEAT1 in human CRC progression. Furthermore, RNA pull-down, mass spectrometry, RNA immunoprecipitation, and Dual-Luciferase Reporter Assays were used to determine the mechanism of NEAT1 in CRC progression. Animal experiments were used to determine the role of NEAT1 in CRC tumorigenicity and metastasis in vivo.
Results: NEAT1 expression was significantly upregulated in CRC tissues compared with its expression in normal tissues. Altered NEAT1 expression led to marked changes in proliferation, migration, and invasion of CRC cells both in vitro and in vivo. Mechanistically, we found that NEAT1 directly bound to the DDX5 protein, regulated its stability, and sequentially activated Wnt signaling. Our study showed that NEAT1 indirectly activated the Wnt/β-catenin signaling pathway via DDX5 and fulfilled its oncogenic functions in a DDX5-mediated manner. Clinically, concomitant NEAT1 and DDX5 protein levels negatively correlated with the overall survival and disease-free survival of CRC patients.
Conclusions: Our findings indicated that NEAT1 activated Wnt signaling to promote colorectal cancer progression and metastasis. The NEAT1/DDX5/Wnt/β-catenin axis could be a potential therapeutic target of pharmacological strategies.
Keywords: Colorectal cancer; DDX5; NEAT1; lncRNA; β-Catenin.
Conflict of interest statement
Ethics approval
The research protocol was reviewed and approved by the Ethics Committee of FDUSCC, and informed consent was obtained from all participants included in the study in agreement with institutional guidelines. This study complied with the Animal Care guidelines of FDUSCC.
Consent for publication
Informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

