Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis
- PMID: 30185234
- PMCID: PMC6123971
- DOI: 10.1186/s40659-018-0182-7
Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis
Abstract
Background: New evidence demonstrates that aging and dyslipidemia are closely associated with oxidative stress, DNA damage and apoptosis in some cells and extravascular tissues. However, in monocytes, which are naturally involved in progression and/or resolution of plaque in atherosclerosis, this concurrence has not yet been fully investigated. In this study, we evaluated the influence of aging and hypercholesterolemia on serum pro-inflammatory cytokines, oxidative stress, DNA damage and apoptosis in monocytes from apolipoprotein E-deficient (apoE-/-) mice compared with age-matched wild-type C57BL/6 (WT) mice. Experiments were performed in young (2-months) and in old (18-months) male wild-type (WT) and apoE-/- mice.
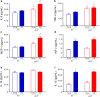
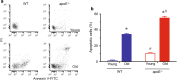
Results: Besides the expected differences in serum lipid profile and plaque formation, we observed that atherosclerotic mice exhibited a significant increase in monocytosis and in serum levels of pro-inflammatory cytokines compared to WT mice. Moreover, it was observed that the overproduction of ROS, led to an increased DNA fragmentation and, consequently, apoptosis in monocytes from normocholesterolemic old mice, which was aggravated in age-matched atherosclerotic mice.
Conclusions: In this study, we demonstrate that a pro-inflammatory systemic status is associated with an impairment of functionality of monocytes during aging and that these parameters are fundamental extra-arterial contributors to the aggravation of atherosclerosis. The present data open new avenues for the development of future strategies with the purpose of treating atherosclerosis.
Keywords: Apoptosis; Atherosclerosis; Proinflammatory cytokines; apoE knockout mice.
Figures




References
-
- Rudolf J, Lewandrowski KB. Cholesterol, lipoproteins, high-sensitivity c-reactive protein, and other risk factors for atherosclerosis. Clin Lab Med. 2014 - PubMed
MeSH terms
Substances
Grants and funding
- 307584/2015-1/Conselho Nacional de Desenvolvimento Científico e Tecnológico (BR)
- 303001/2015-1/Conselho Nacional de Desenvolvimento Científico e Tecnológico (BR)
- 445080/2014-0/Conselho Nacional de Desenvolvimento Científico e Tecnológico (BR)
- 445736/2014-3/Conselho Nacional de Desenvolvimento Científico e Tecnológico (BR)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

