Antiepileptic drug clearances during pregnancy and clinical implications for women with epilepsy
- PMID: 30185446
- PMCID: PMC6161546
- DOI: 10.1212/WNL.0000000000006240
Antiepileptic drug clearances during pregnancy and clinical implications for women with epilepsy
Abstract
Objective: To characterize the magnitude and time course of pregnancy-related clearance changes for different antiepileptic drugs (AEDs): levetiracetam, oxcarbazepine, topiramate, phenytoin, and valproate. A secondary aim was to determine if a decreased AED serum concentration was associated with increased seizure frequency.
Methods: Women with epilepsy were enrolled preconception or early in pregnancy and prospectively followed throughout pregnancy and the first postpartum year with daily diaries of AED doses, adherence, and seizures. Study visits with AED concentration measurements occurred every 1-3 months. AED clearances in each trimester were compared to nonpregnant baseline using a mixed linear regression model, with adjustments for age, race, and hours postdose. In women on monotherapy, 2-sample t test was used to compare the ratio to target concentrations (RTC) between women with seizure worsening each trimester and those without.
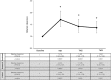
Results: AED clearances were calculated for levetiracetam (n = 18 pregnancies), oxcarbazepine (n = 4), topiramate (n = 10), valproate (n = 5), and phenytoin (n = 7). Mean maximal clearances were reached for (1) levetiracetam in first trimester (1.71-fold baseline clearance) (p = 0.0001), (2) oxcarbazepine in second trimester (1.63-fold) (p = 0.0001), and (3) topiramate in second trimester (1.39-fold) (p = 0.025). In 15 women on AED monotherapy, increased seizure frequency in the first, second, and all trimesters was associated with a lower RTC (p < 0.05).
Conclusion: AED clearance significantly changes by the first trimester for levetiracetam and by the second trimester for oxcarbazepine and topiramate. Lower RTC was associated with seizure worsening. Early therapeutic drug monitoring and dose adjustment may be helpful to avoid increased seizure frequency.
© 2018 American Academy of Neurology.
Figures



References
-
- Harden CL, Pennell PB, Koppel BS, et al. Practice parameter update: management issues for women with epilepsy: focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009;73:142–149. - PMC - PubMed
-
- International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures: from the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1981;22:489–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
