Graded Transmission without Action Potentials Sustains Rhythmic Activity in Some But Not All Modulators That Activate the Same Current
- PMID: 30185461
- PMCID: PMC6191523
- DOI: 10.1523/JNEUROSCI.2632-17.2018
Graded Transmission without Action Potentials Sustains Rhythmic Activity in Some But Not All Modulators That Activate the Same Current
Abstract
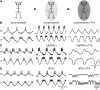
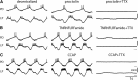
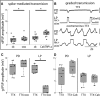
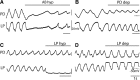
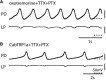
Neurons in the central pattern-generating circuits in the crustacean stomatogastric ganglion (STG) release neurotransmitter both as a graded function of presynaptic membrane potential that persists in TTX and in response to action potentials. In the STG of the male crab Cancer borealis, the modulators oxotremorine, C. borealis tachykinin-related peptide Ia (CabTRP1a), red pigment concentrating hormone (RPCH), proctolin, TNRNFLRFamide, and crustacean cardioactive peptide (CCAP) produce and sustain robust pyloric rhythms by activating the same modulatory current (IMI), albeit on different subsets of pyloric network targets. The muscarinic agonist oxotremorine, and the peptides CabTRP1a and RPCH elicited rhythmic triphasic intracellular alternating fluctuations of activity in the presence of TTX. Intracellular waveforms of pyloric neurons in oxotremorine and CabTRP1a in TTX were similar to those in the intact rhythm, and phase relationships among neurons were conserved. Although cycle frequency was conserved in oxotremorine and TTX, it was altered in CabTRP1a in the presence of TTX. Both rhythms were primarily driven by the pacemaker kernel consisting of the Anterior Burster and Pyloric Dilator neurons. In contrast, in TTX the circuit remained silent in proctolin, TNRNFLRFamide, and CCAP. These experiments show that graded synaptic transmission in the absence of voltage-gated Na+ current is sufficient to sustain rhythmic motor activity in some, but not other, modulatory conditions, even when each modulator activates the same ionic current. This further demonstrates that similar rhythmic motor patterns can be produced by qualitatively different mechanisms, one that depends on the activity of voltage-gated Na+ channels, and one that can persist in their absence.SIGNIFICANCE STATEMENT The pyloric rhythm of the crab stomatogastric ganglion depends both on spike-mediated and graded synaptic transmission. We activate the pyloric rhythm with a wide variety of different neuromodulators, all of which converge on the same voltage-dependent inward current. Interestingly, when action potentials and spike-mediated transmission are blocked using TTX, we find that the muscarinic agonist oxotremorine and the neuropeptide CabTRP1a sustain rhythmic alternations and appropriate phases of activity in the absence of action potentials. In contrast, TTX blocks rhythmic activity in the presence of other modulators. This demonstrates fundamental differences in the burst-generation mechanisms in different modulators that would not be suspected on the basis of their cellular actions at the level of the targeted current.
Keywords: Cancer borealis; Cancer borealis tachykinin-related peptide 1a; oxotremorine; proctolin; red pigment concentrating hormone; stomatogastric ganglion.
Copyright © 2018 the authors 0270-6474/18/388976-13$15.00/0.
Figures



Similar articles
-
Modulators with convergent cellular actions elicit distinct circuit outputs.J Neurosci. 2001 Jun 1;21(11):4050-8. doi: 10.1523/JNEUROSCI.21-11-04050.2001. J Neurosci. 2001. PMID: 11356892 Free PMC article.
-
The pyloric neural circuit of the herbivorous crab Pugettia producta shows limited sensitivity to several neuromodulators that elicit robust effects in more opportunistically feeding decapods.J Exp Biol. 2008 May;211(Pt 9):1434-47. doi: 10.1242/jeb.016998. J Exp Biol. 2008. PMID: 18424677
-
Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit.J Neurosci. 2000 Sep 15;20(18):6752-9. doi: 10.1523/JNEUROSCI.20-18-06752.2000. J Neurosci. 2000. PMID: 10995818 Free PMC article.
-
Neuromodulatory inputs maintain expression of a lobster motor pattern-generating network in a modulation-dependent state: evidence from long-term decentralization in vitro.J Neurosci. 1998 Mar 15;18(6):2212-25. doi: 10.1523/JNEUROSCI.18-06-02212.1998. J Neurosci. 1998. PMID: 9482805 Free PMC article. Review.
-
How can motor systems retain performance over a wide temperature range? Lessons from the crustacean stomatogastric nervous system.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015 Sep;201(9):851-6. doi: 10.1007/s00359-014-0975-2. Epub 2015 Jan 1. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015. PMID: 25552317 Free PMC article. Review.
Cited by
-
Short-term synaptic dynamics control the activity phase of neurons in an oscillatory network.Elife. 2019 Jun 10;8:e46911. doi: 10.7554/eLife.46911. Elife. 2019. PMID: 31180323 Free PMC article.
-
Rapid adaptation to elevated extracellular potassium in the pyloric circuit of the crab, Cancer borealis.J Neurophysiol. 2020 May 1;123(5):2075-2089. doi: 10.1152/jn.00135.2020. Epub 2020 Apr 22. J Neurophysiol. 2020. PMID: 32319837 Free PMC article.
-
The differential contribution of pacemaker neurons to synaptic transmission in the pyloric network of the Jonah crab, Cancer borealis.J Neurophysiol. 2019 Oct 1;122(4):1623-1633. doi: 10.1152/jn.00038.2019. Epub 2019 Aug 14. J Neurophysiol. 2019. PMID: 31411938 Free PMC article.
-
Frequency-Dependent Action of Neuromodulation.eNeuro. 2021 Nov 9;8(6):ENEURO.0338-21.2021. doi: 10.1523/ENEURO.0338-21.2021. Print 2021 Nov-Dec. eNeuro. 2021. PMID: 34593519 Free PMC article.
-
Sulfonylurea Receptor Pharmacology Alters the Performance of Two Central Pattern Generating Circuits in Cancer borealis.Function (Oxf). 2024 Nov 20;5(6):zqae043. doi: 10.1093/function/zqae043. Function (Oxf). 2024. PMID: 39293809 Free PMC article.
References
-
- Arshavsky YuI, Beloozerova IN, Orlovsky GN, Panchin YuV, Pavlova GA (1985) Control of locomotion in marine mollusk clione-limacina. III. On the origin of locomotory rhythm. Exp Brain Res 58:273–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
