Germinant Synergy Facilitates Clostridium difficile Spore Germination under Physiological Conditions
- PMID: 30185513
- PMCID: PMC6126144
- DOI: 10.1128/mSphere.00335-18
Germinant Synergy Facilitates Clostridium difficile Spore Germination under Physiological Conditions
Abstract
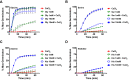
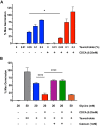
Clostridium difficile is a Gram-positive obligate anaerobe that forms spores in order to survive for long periods in the unfavorable environment outside a host. C. difficile is the leading cause of nosocomial infectious diarrhea worldwide. C. difficile infection (CDI) arises after a patient treated with broad-spectrum antibiotics ingests infectious spores. The first step in C. difficile pathogenesis is the metabolic reactivation of dormant spores within the gastrointestinal (GI) tract through a process known as germination. In this work, we aim to elucidate the specific conditions and the location within the GI tract that facilitate this process. Our data suggest that C. difficile germination occurs through a two-step biochemical process that is regulated by pH and bile salts, amino acids, and calcium present within the GI tract. Maximal germination occurs at a pH ranging from 6.5 to 8.5 in the terminal small intestine prior to bile salt and calcium reabsorption by the host. Germination can be initiated by lower concentrations of germinants when spores are incubated with a combination of bile salts, calcium, and amino acids, and this synergy is dependent on the availability of calcium. The synergy described here allows germination to proceed in the presence of inhibitory bile salts and at physiological concentrations of germinants, effectively decreasing the concentrations of nutrients required to initiate an essential step of pathogenesis.IMPORTANCEClostridium difficile is an anaerobic spore-forming human pathogen that is the leading cause of nosocomial infectious diarrhea worldwide. Germination of infectious spores is the first step in the development of a C. difficile infection (CDI) after ingestion and passage through the stomach. This study investigates the specific conditions that facilitate C. difficile spore germination, including the following: location within the gastrointestinal (GI) tract, pH, temperature, and germinant concentration. The germinants that have been identified in culture include combinations of bile salts and amino acids or bile salts and calcium, but in vitro, these function at concentrations that far exceed normal physiological ranges normally found in the mammalian GI tract. In this work, we describe and quantify a previously unreported synergy observed when bile salts, calcium, and amino acids are added together. These germinant cocktails improve germination efficiency by decreasing the required concentrations of germinants to physiologically relevant levels. Combinations of multiple germinant types are also able to overcome the effects of inhibitory bile salts. In addition, we propose that the acidic conditions within the GI tract regulate C. difficile spore germination and could provide a biological explanation for why patients taking proton pump inhibitors are associated with increased risk of developing a CDI.
Keywords: Clostridium difficile; germination; spore.
Figures




References
-
- Davies KA, Ashwin H, Longshaw CM, Burns DA, Davis GL, Wilcox MH, EUCLID study group. 2016. Diversity of Clostridium difficile PCR ribotypes in Europe: results from the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID), 2012 and 2013. Euro Surveill 21(29):pii=30294. doi: 10.2807/1560-7917.ES.2016.21.29.30294. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

