The Transcription Factor VdHapX Controls Iron Homeostasis and Is Crucial for Virulence in the Vascular Pathogen Verticillium dahliae
- PMID: 30185514
- PMCID: PMC6126142
- DOI: 10.1128/mSphere.00400-18
The Transcription Factor VdHapX Controls Iron Homeostasis and Is Crucial for Virulence in the Vascular Pathogen Verticillium dahliae
Abstract
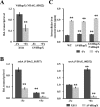
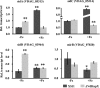
Iron homeostasis is essential for full virulence and viability in many pathogenic fungi. Here, we showed that the bZip transcription factor VdHapX functions as a key regulator of iron homeostasis for adaptation to iron-depleted and iron-excess conditions and is required for full virulence in the vascular wilt fungus, Verticillium dahliae Deletion of VdHapX impaired mycelial growth and conidiation under both iron starvation and iron sufficiency. Furthermore, disruption of VdHapX led to decreased formation of the long-lived survival structures of V. dahliae, known as microsclerotia. Expression of genes involved in iron utilization pathways and siderophore biosynthesis was misregulated in the ΔVdHapX strain under the iron-depleted condition. Additionally, the ΔVdHapX strain exhibited increased sensitivity to high iron concentrations and H2O2, indicating that VdHapX also contributes to iron or H2O2 detoxification. The ΔVdHapX strain showed a strong reduction in virulence on smoke tree seedlings (Cotinus coggygria) and was delayed in its ability to penetrate plant epidermal tissue.IMPORTANCE This study demonstrated that VdHapX is a conserved protein that mediates adaptation to iron starvation and excesses, affects microsclerotium formation, and is crucial for virulence of V. dahliae.
Keywords: HapX; Verticillium dahliae; fungal virulence; iron homeostasis; vascular wilt.
Copyright © 2018 Wang et al.
Figures






References
-
- Lin H, Li L, Jia X, Ward DM, Kaplan J. 2011. Genetic and biochemical analysis of high iron toxicity in yeast: iron toxicity is due to the accumulation of cytosolic iron and occurs under both aerobic and anaerobic conditions. J Biol Chem 286:3851–3862. doi:10.1074/jbc.M110.190959. - DOI - PMC - PubMed
-
- Hortschansky P, Eisendle M, Al-Abdallah Q, Schmidt AD, Bergmann S, Thon M, Kniemeyer O, Abt B, Seeber B, Werner ER, Kato M, Brakhage AA, Haas H. 2007. Interaction of HapX with the CCAAT-binding complex—a novel mechanism of gene regulation by iron. EMBO J 26:3157–3168. doi:10.1038/sj.emboj.7601752. - DOI - PMC - PubMed
-
- Schrettl M, Beckmann N, Varga J, Heinekamp T, Jacobsen ID, Jochl C, Moussa TA, Wang S, Gsaller F, Blatzer M, Werner ER, Niermann WC, Brakhage AA, Haas H. 2010. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog 6:e1001124. doi:10.1371/journal.ppat.1001124. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
