Evaluating the Columnar Stability of Acoustic Processing in the Human Auditory Cortex
- PMID: 30185539
- PMCID: PMC6125808
- DOI: 10.1523/JNEUROSCI.3576-17.2018
Evaluating the Columnar Stability of Acoustic Processing in the Human Auditory Cortex
Abstract
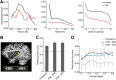
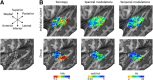
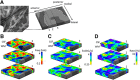
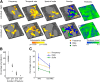
Using ultra-high field fMRI, we explored the cortical depth-dependent stability of acoustic feature preference in human auditory cortex. We collected responses from human auditory cortex (subjects from either sex) to a large number of natural sounds at submillimeter spatial resolution, and observed that these responses were well explained by a model that assumes neuronal population tuning to frequency-specific spectrotemporal modulations. We observed a relatively stable (columnar) tuning to frequency and temporal modulations. However, spectral modulation tuning was variable throughout the cortical depth. This difference in columnar stability between feature maps could not be explained by a difference in map smoothness, as the preference along the cortical sheet varied in a similar manner for the different feature maps. Furthermore, tuning to all three features was more columnar in primary than nonprimary auditory cortex. The observed overall lack of overlapping columnar regions across acoustic feature maps suggests, especially for primary auditory cortex, a coding strategy in which across cortical depths tuning to some features is kept stable, whereas tuning to other features systematically varies.SIGNIFICANCE STATEMENT In the human auditory cortex, sound aspects are processed in large-scale maps. Invasive animal studies show that an additional processing organization may be implemented orthogonal to the cortical sheet (i.e., in the columnar direction), but it is unknown whether observed organizational principles apply to the human auditory cortex. Combining ultra-high field fMRI with natural sounds, we explore the columnar organization of various sound aspects. Our results suggest that the human auditory cortex contains a modular coding strategy, where, for each module, several sound aspects act as an anchor along which computations are performed while the processing of another sound aspect undergoes a transformation. This strategy may serve to optimally represent the content of our complex acoustic natural environment.
Keywords: auditory cortex; columnar processing; spectrotemporal modulations; tonotopy; ultra-high field fMRI.
Copyright © 2018 the authors 0270-6474/18/387822-11$15.00/0.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
