Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models
- PMID: 30185553
- PMCID: PMC6156612
- DOI: 10.1073/pnas.1721136115
Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models
Erratum in
-
Correction for Lee et al., Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models.Proc Natl Acad Sci U S A. 2018 Oct 16;115(42):E9992. doi: 10.1073/pnas.1815900115. Epub 2018 Oct 8. Proc Natl Acad Sci U S A. 2018. PMID: 30297410 Free PMC article. No abstract available.
Abstract
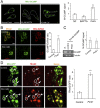
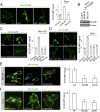
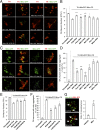
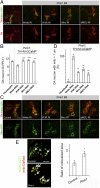
Calcium (Ca2+) homeostasis is essential for neuronal function and survival. Altered Ca2+ homeostasis has been consistently observed in neurological diseases. How Ca2+ homeostasis is achieved in various cellular compartments of disease-relevant cell types is not well understood. Here we show in Drosophila Parkinson's disease (PD) models that Ca2+ transport from the endoplasmic reticulum (ER) to mitochondria through the ER-mitochondria contact site (ERMCS) critically regulates mitochondrial Ca2+ (mito-Ca2+) homeostasis in dopaminergic (DA) neurons, and that the PD-associated PINK1 protein modulates this process. In PINK1 mutant DA neurons, the ERMCS is strengthened and mito-Ca2+ level is elevated, resulting in mitochondrial enlargement and neuronal death. Miro, a well-characterized component of the mitochondrial trafficking machinery, mediates the effects of PINK1 on mito-Ca2+ and mitochondrial morphology, apparently in a transport-independent manner. Miro overexpression mimics PINK1 loss-of-function effect, whereas inhibition of Miro or components of the ERMCS, or pharmacological modulation of ERMCS function, rescued PINK1 mutant phenotypes. Mito-Ca2+ homeostasis is also altered in the LRRK2-G2019S model of PD and the PAR-1/MARK model of neurodegeneration, and genetic or pharmacological restoration of mito-Ca2+ level is beneficial in these models. Our results highlight the importance of mito-Ca2+ homeostasis maintained by Miro and the ERMCS to mitochondrial physiology and neuronal integrity. Targeting this mito-Ca2+ homeostasis pathway holds promise for a therapeutic strategy for neurodegenerative diseases.
Keywords: ER–mitochondria contact site; Miro; PINK1; Parkinson’s disease; calcium homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
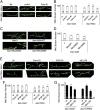
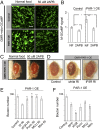
References
-
- Chan DC. Mitochondria: Dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

