Study protocol for a single-blind, randomised controlled, non-inferiority trial of internet-based versus face-to-face cognitive behaviour therapy for obsessive-compulsive disorder
- PMID: 30185575
- PMCID: PMC6129083
- DOI: 10.1136/bmjopen-2018-022254
Study protocol for a single-blind, randomised controlled, non-inferiority trial of internet-based versus face-to-face cognitive behaviour therapy for obsessive-compulsive disorder
Abstract
Introduction: Expert guidelines recommend cognitive-behavioural therapy (CBT) as a first-line treatment for obsessive-compulsive disorder (OCD), but the majority of patients with OCD do not have access to CBT. Internet-delivered CBT (ICBT) has the potential to make this evidence-based treatment more accessible while requiring less therapist time than traditional face-to-face (f2f) CBT. Data from six clinical trials suggest that ICBT for OCD is both efficacious and cost-effective, but whether ICBT is non-inferior to traditional f2f CBT for OCD is yet unknown.
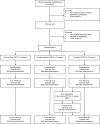
Methods and analysis: A single-blind, randomised, controlled, non-inferiority trial comparing therapist-guided ICBT, unguided ICBT and individual (f2f) CBT for adult OCD patients. The primary objective is to investigate whether ICBT is non-inferior to gold standard f2f CBT. Secondary objectives are to investigate if ICBT is equally effective when delivered unguided, to establish the cost-effectiveness of ICBT and to investigate if the treatment outcome differs between self-referred and clinically referred patients. Participants will be recruited at two specialist OCD clinics in Stockholm and also through online self-referral. Participants will be randomised to one of three treatment conditions: F2f CBT, ICBT with therapist support or unguided ICBT. The total number of participants will be 120, and masked assessments will be administered at baseline, biweekly during treatment, at post-treatment and at 3-month and 12-month follow-ups. The main outcome measure is the clinician-rated Yale-Brown Obsessive Compulsive Scale (Y-BOCS) at 3-month follow-up. The margin of non-inferiority is set to 3 points on the Y-BOCS using a 90% CI.
Ethics and dissemination: The study has been approved by the Regional Ethics Board of Stockholm (REPN 2015/1099-31/2) and registered at Clinicaltrials.gov (NCT02541968). The study will be reported in accordance with the Consolidated Standards of Reporting Trials statement for non-pharmacological trials. The results will be published in peer-reviewed academic journals and disseminated to patient organisations and media.
Trial registration number: NCT02541968; Pre-results.
Keywords: adult psychiatry; clinical randomized controlled trial; internet delivered cognitive behaviour theraphy; non-inferiority study; obsessive compulsive disorder.
© Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Effect of Internet-Based vs Face-to-Face Cognitive Behavioral Therapy for Adults With Obsessive-Compulsive Disorder: A Randomized Clinical Trial.JAMA Netw Open. 2022 Mar 1;5(3):e221967. doi: 10.1001/jamanetworkopen.2022.1967. JAMA Netw Open. 2022. PMID: 35285923 Free PMC article. Clinical Trial.
-
Therapist-Assisted Internet-Based Cognitive Behavioral Therapy Versus Progressive Relaxation in Obsessive-Compulsive Disorder: Randomized Controlled Trial.J Med Internet Res. 2018 Aug 8;20(8):e242. doi: 10.2196/jmir.9566. J Med Internet Res. 2018. PMID: 30089607 Free PMC article. Clinical Trial.
-
Study protocol for a randomised controlled trial of internet-based cognitive-behavioural therapy for obsessive-compulsive disorder.BMC Psychiatry. 2014 Jul 25;14:209. doi: 10.1186/1471-244X-14-209. BMC Psychiatry. 2014. PMID: 25062747 Free PMC article. Clinical Trial.
-
Acceptability, feasibility, and efficacy of Internet cognitive behavioral therapy (iCBT) for pediatric obsessive-compulsive disorder: a systematic review.Syst Rev. 2019 Nov 20;8(1):284. doi: 10.1186/s13643-019-1166-6. Syst Rev. 2019. PMID: 31747935 Free PMC article.
-
Efficacy and acceptability of cognitive-behavioral therapy and serotonin reuptake inhibitors for pediatric obsessive-compulsive disorder: a network meta-analysis.J Child Psychol Psychiatry. 2024 May;65(5):594-609. doi: 10.1111/jcpp.13934. Epub 2024 Jan 3. J Child Psychol Psychiatry. 2024. PMID: 38171647 Review.
Cited by
-
Comparing Effectiveness Between a Mobile App Program and Traditional Cognitive Behavior Therapy in Obsessive-Compulsive Disorder: Evaluation Study.JMIR Ment Health. 2021 Jan 19;8(1):e23778. doi: 10.2196/23778. JMIR Ment Health. 2021. PMID: 33464208 Free PMC article.
-
Randomized trial of the efficacy of trial-based cognitive therapy for obsessive-compulsive disorder: preliminary findings.Trends Psychiatry Psychother. 2023;45:e20210247. doi: 10.47626/2237-6089-2021-0247. Epub 2022 May 25. Trends Psychiatry Psychother. 2023. PMID: 35500249 Free PMC article. Clinical Trial.
-
Innovations in the Delivery of Exposure and Response Prevention for Obsessive-Compulsive Disorder.Curr Top Behav Neurosci. 2021;49:301-329. doi: 10.1007/7854_2020_202. Curr Top Behav Neurosci. 2021. PMID: 33590457
-
Acceptability, feasibility, and effectiveness of internet-based cognitive behavior therapy for obsessive-compulsive disorder (OCD-NET): a naturalistic pilot trial during the COVID-19 pandemic in a psychiatric outpatient department in Germany.BMC Psychiatry. 2025 Jan 30;25(1):85. doi: 10.1186/s12888-025-06519-7. BMC Psychiatry. 2025. PMID: 39885463 Free PMC article.
-
Internet-based psychotherapy in children with obsessive-compulsive disorder (OCD): protocol of a randomized controlled trial.Trials. 2022 Feb 21;23(1):164. doi: 10.1186/s13063-022-06062-w. Trials. 2022. PMID: 35189937 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical