Relative importance of informational items in participant information leaflets for trials: a Q-methodology approach
- PMID: 30185580
- PMCID: PMC6129101
- DOI: 10.1136/bmjopen-2018-023303
Relative importance of informational items in participant information leaflets for trials: a Q-methodology approach
Abstract
Objectives: To identify which information items potential participants and research nurses rank as the most important, and the reasons for this, when considering participation in a randomised controlled trial.
Design: Q-methodology approach alongside a think-aloud process. Using a vignette outlining a hypothetical trial, participants were asked to rank statements about informational items usually included in a participant information leaflet (PIL) on a Q-grid, while undertaking a real-time think-aloud process to elicit the underpinning decision processes. Analysis of quantitative data was conducted using descriptive statistics and qualitative data was coded using content analysis.
Participants: 20 participants (10 potential trial participants and 10 research nurses).
Setting: UK-based participants.
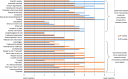
Results: Ten research nurses and 10 potential trial participants provided data for the study. Both stakeholder groups ranked similar statements in their top three most important statements, with 'What are the possible disadvantages and risks of taking part?' featuring in both. However, considerable variability existed between the groups with regard to their ranking of statements of least importance. Participants identified that sufficient information to make a decision was secured using around 14 items. Participants also identified other items of importance not routinely included in PILs.
Conclusions: This study has provided a unique insight into how and why different trial stakeholder groups rank informational items currently contained within PILs. These results have implications for those developing future PILs and those who develop guidance on their content; PILs should focus most on the information items that potential trial participants want and need to make an informed choice about trial participation.
Keywords: Q-methodology; informed consent; participant information leaflets; randomised controlled trials.
© Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Pocock SJ. Clinical trials - a practical approach. New Jersey: John Wiley & Sons Ltd, 1983.
-
- NHS Choices. Clinical Trials and medical research - Ethics committees. http://www.nhs.uk/conditions/clincial-trials/Pages/Ethicscommittees.aspx (accessed 09 Mar 2018).
-
- WMA - World Medical Association. WMA Declaration of Helsinki - ethical principles for medical research involving human subjects. http://www.wma.net/en/30publications/10policies/b3/ (accessed 09 Mar 2018). - PubMed
-
- EU directive 2011/20/EC. 2004. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/reg_... (accessed 09 Mar 2018).
-
- NHS. Informing participants and seeking consent. https://www.hra.nhs.uk/planning-and-improving-research/best-practice/inf... (accessed 09 Mar 2018).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources