Qualitative Differences in Capsidless L-Particles Released as a By-Product of Bovine Herpesvirus 1 and Herpes Simplex Virus 1 Infections
- PMID: 30185590
- PMCID: PMC6206470
- DOI: 10.1128/JVI.01259-18
Qualitative Differences in Capsidless L-Particles Released as a By-Product of Bovine Herpesvirus 1 and Herpes Simplex Virus 1 Infections
Abstract
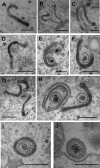
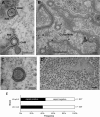
Despite differences in the pathogenesis and host range of alphaherpesviruses, many stages of their morphogenesis are thought to be conserved. Here, an ultrastructural study of bovine herpesvirus 1 (BoHV-1) envelopment revealed profiles similar to those previously found for herpes simplex virus 1 (HSV-1), with BoHV-1 capsids associating with endocytic tubules. Consistent with the similarity of their genomes and envelopment strategies, the proteomic compositions of BoHV-1 and HSV-1 virions were also comparable. However, BoHV-1 morphogenesis exhibited a diversity in envelopment events. First, heterogeneous primary envelopment profiles were readily detectable at the inner nuclear membrane of BoHV-1-infected cells. Second, the BoHV-1 progeny comprised not just full virions but also an abundance of capsidless, noninfectious light particles (L-particles) that were released from the infected cells in numbers similar to those of virions and in the absence of DNA replication. Proteomic analysis of BoHV-1 L-particles and the much less abundant HSV-1 L-particles revealed that they contained the same complement of envelope proteins as virions but showed variations in tegument content. In the case of HSV-1, the UL46 tegument protein was reproducibly found to be >6-fold enriched in HSV-1 L-particles. More strikingly, the tegument proteins UL36, UL37, UL21, and UL16 were depleted in BoHV-1 but not HSV-1 L-particles. We propose that these combined differences reflect the presence of truly segregated "inner" and "outer" teguments in BoHV-1, making it a critical system for studying the structure and process of tegumentation and envelopment.IMPORTANCE The alphaherpesvirus family includes viruses that infect humans and animals. Hence, not only do they have a significant impact on human health, but they also have a substantial economic impact on the farming industry. While the pathogenic manifestations of the individual viruses differ from host to host, their relative genetic compositions suggest similarity at the molecular level. This study provides a side-by-side comparison of the particle outputs from the major human pathogen HSV-1 and the veterinary pathogen BoHV-1. Ultrastructural and proteomic analyses have revealed that both viruses have broadly similar morphogenesis profiles and infectious virus compositions. However, the demonstration that BoHV-1 has the capacity to generate vast numbers of capsidless enveloped particles that differ from those produced by HSV-1 in composition implies a divergence in the cell biology of these viruses that impacts our general understanding of alphaherpesvirus morphogenesis.
Keywords: BoHV-1; HSV-1; L-particles; envelopment; morphogenesis; tegument.
Copyright © 2018 Russell et al.
Figures




References
-
- Bennett R. 2003. The ‘direct costs’ of livestock disease: the development of a system of models for the analysis of 30 endemic livestock diseases in Great Britain. J Agric Econ 54:55–71. doi: 10.1111/j.1477-9552.2003.tb00048.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

