Potent Neutralizing Human Monoclonal Antibodies Preferentially Target Mature Dengue Virus Particles: Implication for Novel Strategy for Dengue Vaccine
- PMID: 30185598
- PMCID: PMC6232466
- DOI: 10.1128/JVI.00556-18
Potent Neutralizing Human Monoclonal Antibodies Preferentially Target Mature Dengue Virus Particles: Implication for Novel Strategy for Dengue Vaccine
Abstract
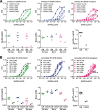
The four serotypes of dengue virus (DENV) cause the most important mosquito-borne viral disease in humans. The envelope (E) protein is the major target of neutralizing antibodies and contains 3 domains (domain I [DI], DII, and DIII). Recent studies reported that human monoclonal antibodies (MAbs) recognizing DIII, the D1/DII hinge, the E-dimer epitope, or a quaternary epitope involving DI/DII/DIII are more potently neutralizing than those recognizing the fusion loop (FL) of DII. Due to inefficient cleavage of the premembrane protein, DENV suspensions consist of a mixture of mature, immature, and partially immature particles. We investigated the neutralization and binding of 22 human MAbs to DENV serotype 1 (DENV1) virions with differential maturation status. Compared with FL MAbs, DIII, DI/DII hinge, and E-dimer epitope MAbs showed higher maximum binding and avidity to mature particles relative to immature particles; this feature may contribute to the strong neutralizing potency of such MAbs. FL-specific MAbs required 57 to 87% occupancy on mature particles to achieve half-maximal neutralization (NT50), whereas the potently neutralizing MAbs achieved NT50 states at 20 to 38% occupancy. Analysis of the MAb repertoire and polyclonal sera from patients with primary DENV1 infection supports the immunodominance of cross-reactive anti-E antibodies over type-specific antibodies. After depletion with viral particles from a heterologous DENV serotype, the type-specific neutralizing antibodies remained and showed binding features shared by potent neutralizing MAbs. Taken together, these findings suggest that the use of homogeneous mature DENV particles as an immunogen may induce more potent neutralizing antibodies against DENV than the use of immature or mixed particles.IMPORTANCE With an estimated 390 million infections per year, the four serotypes of dengue virus (DENV) cause the most important mosquito-borne viral disease in humans. The dengue vaccine Dengvaxia was licensed; however, its low efficacy among dengue-naive individuals and increased risk of causing severe dengue in children highlight the need for a better understanding of the role of human antibodies in immunity against DENV. DENV suspensions contain mature, immature, and partially immature particles. We investigated the binding of 22 human monoclonal antibodies (MAbs) to the DENV envelope protein on particles with different maturation states. Potently neutralizing MAbs had higher relative maximum binding and avidity to mature particles than weakly neutralizing MAbs. This was supported by analysis of MAb repertoires and polyclonal sera from patients with primary DENV infection. Together, these findings suggest that mature particles may be the optimal form of presentation of the envelope protein to induce more potent neutralizing antibodies against DENV.
Keywords: dengue virus; envelope; mature particles; monoclonal antibody; neutralization.
Copyright © 2018 American Society for Microbiology.
Figures






References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. - DOI - PMC - PubMed
-
- World Health Organization. 2009. Dengue hemorrhagic fever: diagnosis, treatment, prevention and control, 3rd ed World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

