Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention
- PMID: 30185661
- PMCID: PMC6171797
- DOI: 10.1172/jci.insight.121326
Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention
Abstract
Background: Plasma lipidomic measures may enable improved prediction of cardiovascular outcomes in secondary prevention. The aim of this study is to determine the association of plasma lipidomic measurements with cardiovascular events and assess their potential to predict such events.
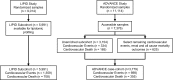
Methods: Plasma lipids (n = 342) were measured in a retrospective subcohort (n = 5,991) of the LIPID study. Proportional hazards regression was used to identify lipids associated with future cardiovascular events (nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death) and cardiovascular death. Multivariable models adding lipid species to traditional risk factors were created using lipid ranking established from the Akaike information criterion within a 5-fold cross-validation framework. The results were tested on a diabetic case cohort from the ADVANCE study (n = 3,779).
Results: Specific ceramide species, sphingolipids, phospholipids, and neutral lipids containing omega-6 fatty acids or odd-chain fatty acids were associated with future cardiovascular events (106 species) and cardiovascular death (139 species). The addition of 7 lipid species to a base model (11 conventional risk factors) resulted in an increase in the C-statistics from 0.629 (95% CI, 0.628-0.630) to 0.654 (95% CI, 0.653-0.656) for prediction of cardiovascular events and from 0.673 (95% CI, 0.671-0.675) to 0.727 (95% CI, 0.725-0.728) for prediction of cardiovascular death. Categorical net reclassification improvements for cardiovascular events and cardiovascular death were 0.083 (95% CI, 0.081-0.086) and 0.166 (95% CI, 0.162-0.170), respectively. Evaluation on the ADVANCE case cohort demonstrated significant improvement on the base models.
Conclusions: The improvement in the prediction of cardiovascular outcomes, above conventional risk factors, demonstrates the potential of plasma lipidomic profiles as biomarkers for cardiovascular risk stratification in secondary prevention.
Funding: Bristol-Myers Squibb, the National Health and Medical Research Council of Australia (grants 211086, 358395, and 1029754), and the Operational Infrastructure Support Program of the Victorian government of Australia.
Keywords: Atherosclerosis; Cardiology; Cardiovascular disease; Metabolism.
Conflict of interest statement
Figures




References
-
- Puri R, et al. C-reactive protein, but not low-density lipoprotein cholesterol levels, associate with coronary atheroma regression and cardiovascular events after maximally intensive statin therapy. Circulation. 2013;128(22):2395–2403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

