Polygenic risk score for predicting weight loss after bariatric surgery
- PMID: 30185664
- PMCID: PMC6171810
- DOI: 10.1172/jci.insight.122011
Polygenic risk score for predicting weight loss after bariatric surgery
Abstract
Background: The extent of weight loss among patients undergoing bariatric surgery is highly variable. Herein, we tested the contribution of genetic background to such interindividual variability after biliopancreatic diversion with duodenal switch.
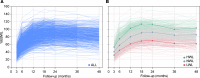
Methods: Percentage of excess body weight loss (%EBWL) was monitored in 865 patients over a period of 48 months after bariatric surgery, and two polygenic risk scores were constructed with 186 and 11 (PRS186 and PRS11) single nucleotide polymorphisms previously associated with body mass index (BMI).
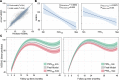
Results: The accuracy of the %EBWL logistic prediction model - including initial BMI, age, sex, and surgery modality, and assessed as the area under the receiver operating characteristics (ROC) curve adjusted for optimism (AUCadj = 0.867) - significantly increased after the inclusion of PRS186 (ΔAUCadj = 0.021; 95% CI of the difference [95% CIdiff] = 0.005-0.038) but not PRS11 (ΔAUCadj= 0.008; 95% CIdiff= -0.003-0.019). The overall fit of the longitudinal linear mixed model for %EBWL showed a significant increase after addition of PRS186 (-2 log-likelihood = 12.3; P = 0.002) and PRS11 (-2 log-likelihood = 9.9; P = 0.007). A significant interaction with postsurgery time was found for PRS186 (β = -0.003; P = 0.008) and PRS11 (β = -0.008; P = 0.03). The inclusion of PRS186 and PRS11 in the model improved the cost-effectiveness of bariatric surgery by reducing the percentage of false negatives from 20.4% to 10.9% and 10.2%, respectively.
Conclusion: These results revealed that genetic background has a significant impact on weight loss after biliopancreatic diversion with duodenal switch. Likewise, the improvement in weight loss prediction after addition of polygenic risk scores is cost-effective, suggesting that genetic testing could potentially be used in the presurgical assessment of patients with severe obesity.
Funding: Heart and Stroke Foundation of Canada (G-17-0016627) and Canada Research Chair in Genomics Applied to Nutrition and Metabolic Health (no. 950-231-580).
Keywords: Genetics; Obesity; Surgery.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

