Lysyl oxidase (LOX) limits VSMC proliferation and neointimal thickening through its extracellular enzymatic activity
- PMID: 30185869
- PMCID: PMC6125287
- DOI: 10.1038/s41598-018-31312-w
Lysyl oxidase (LOX) limits VSMC proliferation and neointimal thickening through its extracellular enzymatic activity
Abstract
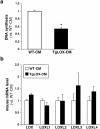
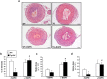
Lysyl oxidase (LOX) plays a critical role in extracellular matrix maturation and limits VSMC proliferation and vascular remodeling. We have investigated whether this anti-proliferative effect relies on the extracellular catalytically active LOX or on its biologically active propeptide (LOX-PP). High expression levels of both LOX and LOX-PP were detected in the vascular wall from transgenic mice over-expressing the full-length human LOX cDNA under the control of SM22α promoter (TgLOX), which targets the transgene to VSMC without affecting the expression of mouse LOX isoenzymes. TgLOX VSMC also secrete high amounts of both mature LOX and LOX-PP. Wild-type (WT) mouse VSMC exposed to VSMC supernatants from transgenic animals showed reduced proliferative rates (low [3H]-thymidine uptake and expression of PCNA) than those incubated with conditioned media from WT cells, effect that was abrogated by β-aminopropionitrile (BAPN), an inhibitor of LOX activity. Lentiviral over-expression of LOX, but not LOX-PP, decreased human VSMC proliferation, effect that was also prevented by BAPN. LOX transgenesis neither impacted local nor systemic inflammatory response induced by carotid artery ligation. Interestingly, in this model, BAPN normalized the reduced neointimal thickening observed in TgLOX mice. Therefore, extracellular enzymatically active LOX is required to limit both VSMC proliferation and vascular remodeling.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Lysyl oxidase (LOX) in vascular remodelling. Insight from a new animal model.Thromb Haemost. 2014 Oct;112(4):812-24. doi: 10.1160/TH14-01-0024. Epub 2014 Jul 3. Thromb Haemost. 2014. PMID: 24990180
-
Inhibition of enzymes involved in collagen cross-linking reduces vascular smooth muscle cell calcification.FASEB J. 2018 Aug;32(8):4459-4469. doi: 10.1096/fj.201700653R. Epub 2018 Mar 16. FASEB J. 2018. PMID: 29547702
-
Lysyl oxidase expression in smooth muscle cells determines the level of intima calcification in hypercholesterolemia-induced atherosclerosis.Clin Investig Arterioscler. 2024 Sep-Oct;36(5):286-298. doi: 10.1016/j.arteri.2024.01.003. Epub 2024 Feb 23. Clin Investig Arterioscler. 2024. PMID: 38402026 English, Spanish.
-
Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases.Cardiovasc Res. 2008 Jul 1;79(1):7-13. doi: 10.1093/cvr/cvn102. Epub 2008 May 9. Cardiovasc Res. 2008. PMID: 18469024 Review.
-
Emerging Roles of Lysyl Oxidases in the Cardiovascular System: New Concepts and Therapeutic Challenges.Biomolecules. 2019 Oct 14;9(10):610. doi: 10.3390/biom9100610. Biomolecules. 2019. PMID: 31615160 Free PMC article. Review.
Cited by
-
Deciphering the Role of Copper Homeostasis in Atherosclerosis: From Molecular Mechanisms to Therapeutic Targets.Int J Mol Sci. 2024 Oct 25;25(21):11462. doi: 10.3390/ijms252111462. Int J Mol Sci. 2024. PMID: 39519014 Free PMC article. Review.
-
Recombinant lysyl oxidase effects on embryonic tendon cell phenotype and behavior.J Orthop Res. 2023 Oct;41(10):2175-2185. doi: 10.1002/jor.25655. Epub 2023 Jul 10. J Orthop Res. 2023. PMID: 37365857 Free PMC article.
-
Total Panax notoginseng saponin inhibits balloon injury-induced neointimal hyperplasia in rat carotid artery models by suppressing pERK/p38 MAPK pathways.Braz J Med Biol Res. 2019 Dec 20;53(1):e9085. doi: 10.1590/1414-431X20199085. eCollection 2020. Braz J Med Biol Res. 2019. PMID: 31859914 Free PMC article.
-
Upregulation of Lysyl Oxidase Expression in Vitreous of Diabetic Subjects: Implications for Diabetic Retinopathy.Cells. 2019 Sep 21;8(10):1122. doi: 10.3390/cells8101122. Cells. 2019. PMID: 31546618 Free PMC article.
-
Extracellular matrix in vascular homeostasis and disease.Nat Rev Cardiol. 2025 May;22(5):333-353. doi: 10.1038/s41569-024-01103-0. Epub 2025 Jan 2. Nat Rev Cardiol. 2025. PMID: 39743560 Review.
References
-
- Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ. Res. 2002;90:251–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

