Peptidyl arginine deiminase 2 (Padi2) is expressed in Sertoli cells in a specific manner and regulated by SOX9 during testicular development
- PMID: 30185873
- PMCID: PMC6125343
- DOI: 10.1038/s41598-018-31376-8
Peptidyl arginine deiminase 2 (Padi2) is expressed in Sertoli cells in a specific manner and regulated by SOX9 during testicular development
Abstract
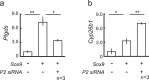
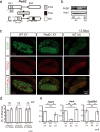
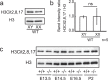
Peptidyl arginine deiminases (PADIs) are enzymes that change the charge of proteins through citrullination. We recently found Padi2 was expressed exclusively in fetal Sertoli cells. In this study, we analyzed the transcriptional regulation of Padi2 and the role of PADI2 in testicular development. We showed SOX9 positively regulated Padi2 transcription and FOXL2 antagonized it in TM3 cells, a model of Sertoli cells. The responsive region to SOX9 and FOXL2 was identified within the Padi2 sequence by reporter assay. In fetal testes from Sox9 knockout (AMH-Cre:Sox9flox/flox) mice, Padi2 expression was greatly reduced, indicating SOX9 regulates Padi2 in vivo. In vitro analysis using siRNA suggested PADI2 modified transcriptional regulation by SOX9. However, Padi2-/- XY mice were fertile and showed no apparent reproductive anomalies. Although, PADI2 is known as an epigenetic transcriptional regulator through H3 citrullination, no significant difference in H3 citrullination between wildtype and Padi2-/- XY gonads was observed. These results suggest Padi2 is a novel gene involved in testis development that is specifically expressed in Sertoli cells through the regulation by SOX9 and FOXL2 and PADI2 supports regulation of target genes by SOX9. Analysis of the Padi2-/- XY phenotype suggested a redundant factor compensated for PADI2 function in testicular development.
Conflict of interest statement
Dr. Kashimada’s work has been funded by Japan Society for the Promotion of Science (JSPS). Other authors have no financial or personal relations that could pose a conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

