Protective effect of 1950 MHz electromagnetic field in human neuroblastoma cells challenged with menadione
- PMID: 30185877
- PMCID: PMC6125585
- DOI: 10.1038/s41598-018-31636-7
Protective effect of 1950 MHz electromagnetic field in human neuroblastoma cells challenged with menadione
Abstract
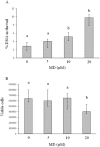
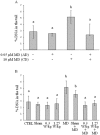
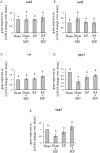
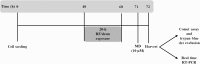
This study aims to assess whether a 1950 MHz radiofrequency (RF) electromagnetic field could protect human neuroblastoma SH-SY5Y cells against a subsequent treatment with menadione, a chemical agent inducing DNA damage via reactive oxygen species formation. Cells were pre-exposed for 20 h to specific absorption rate of either 0.3 or 1.25 W/kg, and 3 h after the end of the exposure, they were treated with 10 µM menadione (MD) for 1 h. No differences were observed between sham- and RF-exposed samples. A statistically significant reduction in menadione-induced DNA damage was detected in cells pre-exposed to either 0.3 or 1.25 W/kg (P < 0.05). Moreover, our analyses of gene expression revealed that the pre-exposure to RF almost inhibited the dramatic loss of glutathione peroxidase-based antioxidant scavenging efficiency that was induced by MD, and in parallel strongly enhanced the gene expression of catalase-based antioxidant protection. In addition, RF abolished the MD-dependent down-regulation of oxoguanine DNA glycosylase, which is a critical DNA repairing enzyme. Overall, our findings suggested that RF pre-exposure reduced menadione-dependent DNA oxidative damage, most probably by enhancing antioxidant scavenging efficiency and restoring DNA repair capability. Our results provided some insights into the molecular mechanisms underlying the RF-induced adaptive response in human neuroblastoma cells challenged with menadione.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Inhibition of Autophagy Negates Radiofrequency-Induced Adaptive Response in SH-SY5Y Neuroblastoma Cells.Int J Mol Sci. 2022 Jul 29;23(15):8414. doi: 10.3390/ijms23158414. Int J Mol Sci. 2022. PMID: 35955556 Free PMC article.
-
Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation.Mutat Res. 2009 Mar 9;662(1-2):54-8. doi: 10.1016/j.mrfmmm.2008.12.005. Epub 2008 Dec 24. Mutat Res. 2009. PMID: 19135463
-
Combined effects of 872 MHz radiofrequency radiation and ferrous chloride on reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells.Bioelectromagnetics. 2010 Sep;31(6):417-24. doi: 10.1002/bem.20580. Bioelectromagnetics. 2010. PMID: 20564172
-
Exposure to 1800 MHz radiofrequency radiation induces oxidative damage to mitochondrial DNA in primary cultured neurons.Brain Res. 2010 Jan 22;1311:189-96. doi: 10.1016/j.brainres.2009.10.062. Epub 2009 Oct 30. Brain Res. 2010. PMID: 19879861
-
Comprehensive Review of Quality of Publications and Meta-analysis of Genetic Damage in Mammalian Cells Exposed to Non-Ionizing Radiofrequency Fields.Radiat Res. 2019 Jan;191(1):20-30. doi: 10.1667/RR15117.1. Epub 2018 Oct 19. Radiat Res. 2019. PMID: 30339042 Review.
Cited by
-
Label-Free Study of the Global Cell Behavior during Exposure to Environmental Radiofrequency Fields in the Presence or Absence of Pro-Apoptotic or Pro-Autophagic Treatments.Int J Mol Sci. 2022 Jan 8;23(2):658. doi: 10.3390/ijms23020658. Int J Mol Sci. 2022. PMID: 35054844 Free PMC article.
-
Investigation of Dalea parryi (Fabaceae) metabolites for anthelmintic activity against the human pathogenic hookworm Ancylostoma ceylanicum.Phytochemistry. 2020 Sep;177:112423. doi: 10.1016/j.phytochem.2020.112423. Epub 2020 Jul 18. Phytochemistry. 2020. PMID: 32688268 Free PMC article.
-
Inhibition of Autophagy Negates Radiofrequency-Induced Adaptive Response in SH-SY5Y Neuroblastoma Cells.Int J Mol Sci. 2022 Jul 29;23(15):8414. doi: 10.3390/ijms23158414. Int J Mol Sci. 2022. PMID: 35955556 Free PMC article.
-
Vitamin K2 Modulates Organelle Damage and Tauopathy Induced by Streptozotocin and Menadione in SH-SY5Y Cells.Antioxidants (Basel). 2021 Jun 20;10(6):983. doi: 10.3390/antiox10060983. Antioxidants (Basel). 2021. PMID: 34202933 Free PMC article.
-
Molecular Relationships in Biofilm Formation and the Biosynthesis of Exoproducts in Pseudoalteromonas spp.Mar Biotechnol (NY). 2022 Jun;24(3):431-447. doi: 10.1007/s10126-022-10097-0. Epub 2022 Apr 29. Mar Biotechnol (NY). 2022. PMID: 35486299 Review.
References
-
- Dimova EG, Bryant PE, Chankova SG. “Adaptive response” - some underlying mechanisms and open questions. Genet Mol Biol. 2008;31:13. doi: 10.1590/S1415-47572008000300002. - DOI
-
- Sasaki, M. S. et al. DNA damage response pathway in radioadaptive response. Mutation research504, 10.1016/s0027-5107(02)00084-2 (2002). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

