Small-scale displacement fluctuations of vesicles in fibroblasts
- PMID: 30185883
- PMCID: PMC6125338
- DOI: 10.1038/s41598-018-31656-3
Small-scale displacement fluctuations of vesicles in fibroblasts
Abstract
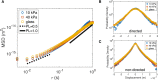
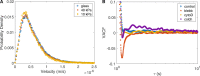
The intracellular environment is a dynamic space filled with various organelles moving in all directions. Included in this diverse group of organelles are vesicles, which are involved in transport of molecular cargo throughout the cell. Vesicles move in either a directed or non-directed fashion, often depending on interactions with cytoskeletal proteins such as microtubules, actin filaments, and molecular motors. How these proteins affect the local fluctuations of vesicles in the cytoplasm is not clear since they have the potential to both facilitate and impede movement. Here we show that vesicle mobility is significantly affected by myosin-II, even though it is not a cargo transport motor. We find that myosin-II activity increases the effective diffusivity of vesicles and its inhibition facilitates longer states of non-directed motion. Our study suggests that altering myosin-II activity in the cytoplasm of cells can modulate the mobility of vesicles, providing a possible mechanism for cells to dynamically tune the cytoplasmic environment in space and time.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

