A homing system targets therapeutic T cells to brain cancer
- PMID: 30185905
- PMCID: PMC6402337
- DOI: 10.1038/s41586-018-0499-y
A homing system targets therapeutic T cells to brain cancer
Retraction in
-
Retraction Note: A homing system targets therapeutic T cells to brain cancer.Nature. 2019 Mar;567(7746):132. doi: 10.1038/s41586-019-0967-z. Nature. 2019. PMID: 30787441 Free PMC article.
Abstract
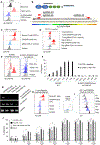
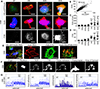
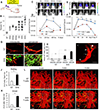
Successful T cell immunotherapy for brain cancer requires that the T cells can access tumour tissues, but this has been difficult to achieve. Here we show that, in contrast to inflammatory brain diseases such as multiple sclerosis, where endothelial cells upregulate ICAM1 and VCAM1 to guide the extravasation of pro-inflammatory cells, cancer endothelium downregulates these molecules to evade immune recognition. By contrast, we found that cancer endothelium upregulates activated leukocyte cell adhesion molecule (ALCAM), which allowed us to overcome this immune-evasion mechanism by creating an ALCAM-restricted homing system (HS). We re-engineered the natural ligand of ALCAM, CD6, in a manner that triggers initial anchorage of T cells to ALCAM and conditionally mediates a secondary wave of adhesion by sensitizing T cells to low-level ICAM1 on the cancer endothelium, thereby creating the adhesion forces necessary to capture T cells from the bloodstream. Cytotoxic HS T cells robustly infiltrated brain cancers after intravenous injection and exhibited potent antitumour activity. We have therefore developed a molecule that targets the delivery of T cells to brain cancer.
Conflict of interest statement
Figures













Comment in
-
T cells engineered to home in on brain cancer.Nature. 2018 Sep;561(7723):319-320. doi: 10.1038/d41586-018-05883-7. Nature. 2018. PMID: 30224728 No abstract available.
-
T cells home in on brain cancer.Nat Rev Immunol. 2018 Oct;18(10):599. doi: 10.1038/s41577-018-0069-4. Nat Rev Immunol. 2018. PMID: 30224770 No abstract available.
-
Steering CAR T Cells into Solid Tumors.N Engl J Med. 2019 Jan 17;380(3):289-291. doi: 10.1056/NEJMcibr1811991. N Engl J Med. 2019. Retraction in: N Engl J Med. 2019 Mar 28;380(13):1286. doi: 10.1056/NEJMc1902526. PMID: 30650328 Retracted. No abstract available.
References
-
- Davenport MP, Grimm MC, and Lloyd AR, A homing selection hypothesis for T-cell trafficking. Immunology Today. 21(7): p. 315–317. - PubMed
-
- Carrithers MD, et al. , Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain, 2000. 123 ( Pt 6): p. 1092–101. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

