The Epigenetic Factor Landscape of Developing Neocortex Is Regulated by Transcription Factors Pax6→ Tbr2→ Tbr1
- PMID: 30186101
- PMCID: PMC6113890
- DOI: 10.3389/fnins.2018.00571
The Epigenetic Factor Landscape of Developing Neocortex Is Regulated by Transcription Factors Pax6→ Tbr2→ Tbr1
Abstract
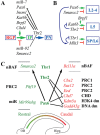
Epigenetic factors (EFs) regulate multiple aspects of cerebral cortex development, including proliferation, differentiation, laminar fate, and regional identity. The same neurodevelopmental processes are also regulated by transcription factors (TFs), notably the Pax6→ Tbr2→ Tbr1 cascade expressed sequentially in radial glial progenitors (RGPs), intermediate progenitors, and postmitotic projection neurons, respectively. Here, we studied the EF landscape and its regulation in embryonic mouse neocortex. Microarray and in situ hybridization assays revealed that many EF genes are expressed in specific cortical cell types, such as intermediate progenitors, or in rostrocaudal gradients. Furthermore, many EF genes are directly bound and transcriptionally regulated by Pax6, Tbr2, or Tbr1, as determined by chromatin immunoprecipitation-sequencing and gene expression analysis of TF mutant cortices. Our analysis demonstrated that Pax6, Tbr2, and Tbr1 form a direct feedforward genetic cascade, with direct feedback repression. Results also revealed that each TF regulates multiple EF genes that control DNA methylation, histone marks, chromatin remodeling, and non-coding RNA. For example, Tbr1 activates Rybp and Auts2 to promote the formation of non-canonical Polycomb repressive complex 1 (PRC1). Also, Pax6, Tbr2, and Tbr1 collectively drive massive changes in the subunit isoform composition of BAF chromatin remodeling complexes during differentiation: for example, a novel switch from Bcl7c (Baf40c) to Bcl7a (Baf40a), the latter directly activated by Tbr2. Of 11 subunits predominantly in neuronal BAF, 7 were transcriptionally activated by Pax6, Tbr2, or Tbr1. Using EFs, Pax6→ Tbr2→ Tbr1 effect persistent changes of gene expression in cell lineages, to propagate features such as regional and laminar identity from progenitors to neurons.
Keywords: BAF; NuRD; cortical development; histone acetylation; lncRNA; microRNA; polycomb; trithorax group.
Figures







Similar articles
-
Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex.J Neurosci. 2005 Jan 5;25(1):247-51. doi: 10.1523/JNEUROSCI.2899-04.2005. J Neurosci. 2005. PMID: 15634788 Free PMC article.
-
Progressive loss of PAX6, TBR2, NEUROD and TBR1 mRNA gradients correlates with translocation of EMX2 to the cortical plate during human cortical development.Eur J Neurosci. 2008 Oct;28(8):1449-56. doi: 10.1111/j.1460-9568.2008.06475.x. Eur J Neurosci. 2008. PMID: 18973570 Free PMC article.
-
Intermediate progenitors and Tbr2 in cortical development.J Anat. 2019 Sep;235(3):616-625. doi: 10.1111/joa.12939. Epub 2019 Jan 24. J Anat. 2019. PMID: 30677129 Free PMC article. Review.
-
Control of Neuronal Development by T-Box Genes in the Brain.Curr Top Dev Biol. 2017;122:279-312. doi: 10.1016/bs.ctdb.2016.08.001. Epub 2016 Sep 1. Curr Top Dev Biol. 2017. PMID: 28057268 Review.
-
Pax6 regulates Tbr1 and Tbr2 expressions in olfactory bulb mitral cells.Mol Cell Neurosci. 2013 May;54:58-70. doi: 10.1016/j.mcn.2013.01.002. Epub 2013 Jan 22. Mol Cell Neurosci. 2013. PMID: 23353076 Free PMC article.
Cited by
-
Transcriptional Regulators and Human-Specific/Primate-Specific Genes in Neocortical Neurogenesis.Int J Mol Sci. 2020 Jun 29;21(13):4614. doi: 10.3390/ijms21134614. Int J Mol Sci. 2020. PMID: 32610533 Free PMC article. Review.
-
Adult Expression of Tbr2 Is Required for the Maintenance but Not Survival of Intrinsically Photosensitive Retinal Ganglion Cells.Front Cell Neurosci. 2022 Mar 23;16:826590. doi: 10.3389/fncel.2022.826590. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35401124 Free PMC article.
-
A TBR1-K228E Mutation Induces Tbr1 Upregulation, Altered Cortical Distribution of Interneurons, Increased Inhibitory Synaptic Transmission, and Autistic-Like Behavioral Deficits in Mice.Front Mol Neurosci. 2019 Oct 9;12:241. doi: 10.3389/fnmol.2019.00241. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31680851 Free PMC article.
-
Evolution of genetic mechanisms regulating cortical neurogenesis.Dev Neurobiol. 2022 Jul;82(5):428-453. doi: 10.1002/dneu.22891. Epub 2022 Jun 22. Dev Neurobiol. 2022. PMID: 35670518 Free PMC article. Review.
-
Oncogenic PAX6 elicits CDK4/6 inhibitor resistance by epigenetically inactivating the LATS2-Hippo signaling pathway.Clin Transl Med. 2021 Aug;11(8):e503. doi: 10.1002/ctm2.503. Clin Transl Med. 2021. PMID: 34459131 Free PMC article.
References
-
- Albert M., Schmitz S. U., Kooistra S. M., Malatesta M., Morales Torres C., Rekling J. C., et al. . (2013). The histone demethylase Jarid1b ensures faithful mouse development by protecting developmental genes from aberrant H3K4me3. PLoS Genet. 9:e1003461. 10.1371/journal.pgen.1003461 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

