Dichotomous Dopaminergic Control of Ventral Pallidum Neurons
- PMID: 30186117
- PMCID: PMC6113373
- DOI: 10.3389/fncel.2018.00260
Dichotomous Dopaminergic Control of Ventral Pallidum Neurons
Abstract
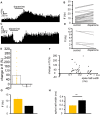
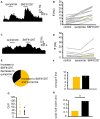
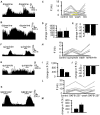
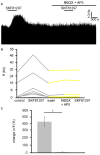
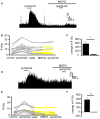
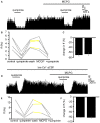
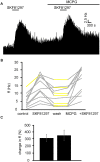
The ventral pallidum (VP) is crucially involved in reward processing. Dopaminergic afferents reach the VP from the ventral tegmental area (VTA). Recent in vivo studies suggest dopamine application increase the firing in the VP. However, little is known about the cellular effects of dopamine within the VP. We aimed to address this paucity of data using brain slices containing the VP and multi-electrode array recordings. Dopamine significantly affected firing in 86% of spontaneously active VP neurons. Among the affected neurons, 84% were excited, while 16% were inhibited. The selective D1-like receptor agonist SKF81297 also had modulatory effects on the majority of VP neurons, but its effects were universally excitatory. On the other hand, the D2-like receptor agonist quinpirole had modulatory effects on 87% of VP neurons studied. It caused significant inhibitory effects in 33% of the cases and excitatory effects in the remaining 67%. The effects of D1-like receptor activation were presynaptic as blocking synaptic transmission with low Ca2+ abolished the effects of SKF81297 application. Furthermore, SKF81297 effects were abolished by blocking ionotropic glutamate receptors, suggesting that D1-like receptors boost glutamate release, which in turn excites VP neurons through postsynaptic glutamate receptors. Effects caused by D2-like receptor activation were found to involve pre and postsynaptic mechanisms, as low Ca2+ abolished the excitatory effects of quinpirole but not the inhibitory ones. Increases in firing frequency (ff) to quinpirole application were abolished by a group 2/3 mGluR antagonist, suggesting that D2-like receptors cause presynaptic inhibition of glutamate release, resulting in reduced postsynaptic activation of inhibitory mGluRs. Conversely, the inhibitory effects of quinpirole persisted in low Ca2+ and therefore can be attributed to postsynaptic D2-like receptor activation. VP neurons excited by dopamine had shorter spike half-widths and are excited by D1-like receptors (presynaptically) and by D2-like receptors (postsynaptically). VP neurons inhibited by dopamine have longer spike half-widths and while D1-like receptor activation has a presynaptic excitatory influence on them, D2-like receptor activation has a postsynaptic inhibitory effect that prevails, on balance. These data provide novel insights into the cellular mechanisms by which dopamine controls information processing within the VP.
Keywords: basal ganglia; dopamine; multielectrode; reward; ventral pallidum.
Figures




Similar articles
-
The D2-like Dopamine Receptor Agonist Quinpirole Microinjected Into the Ventral Pallidum Dose-Dependently Inhibits the VTA and Induces Place Aversion.Int J Neuropsychopharmacol. 2022 Aug 4;25(7):590-599. doi: 10.1093/ijnp/pyac024. Int J Neuropsychopharmacol. 2022. PMID: 35348731 Free PMC article.
-
Dopamine D2 receptor desensitization by dopamine or corticotropin releasing factor in ventral tegmental area neurons is associated with increased glutamate release.Neuropharmacology. 2014 Jul;82:28-40. doi: 10.1016/j.neuropharm.2014.03.006. Epub 2014 Mar 19. Neuropharmacology. 2014. PMID: 24657149 Free PMC article.
-
D1 dopamine receptor stimulation enables the postsynaptic, but not autoreceptor, effects of D2 dopamine agonists in nigrostriatal and mesoaccumbens dopamine systems.Synapse. 1989;4(4):327-46. doi: 10.1002/syn.890040409. Synapse. 1989. PMID: 2532422
-
Bidirectional dopaminergic modulation of excitatory synaptic transmission in orexin neurons.J Neurosci. 2006 Sep 27;26(39):10043-50. doi: 10.1523/JNEUROSCI.1819-06.2006. J Neurosci. 2006. Retraction in: J Neurosci. 2012 Jun 27;32(26):9116. doi: 10.1523/JNEUROSCI.1889-12.2012. PMID: 17005867 Free PMC article. Retracted.
-
Dopamine D1 and D2 receptor agonists induce opposite changes in the firing rate of ventral pallidal neurons.Eur J Pharmacol. 1991 Jul 23;200(1):103-12. doi: 10.1016/0014-2999(91)90672-d. Eur J Pharmacol. 1991. PMID: 1685119
Cited by
-
Effects of Electrical Stimulation of NAc Afferents on VP Neurons' Tonic Firing.Front Cell Neurosci. 2020 Nov 23;14:599920. doi: 10.3389/fncel.2020.599920. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33328895 Free PMC article.
-
The D2-like Dopamine Receptor Agonist Quinpirole Microinjected Into the Ventral Pallidum Dose-Dependently Inhibits the VTA and Induces Place Aversion.Int J Neuropsychopharmacol. 2022 Aug 4;25(7):590-599. doi: 10.1093/ijnp/pyac024. Int J Neuropsychopharmacol. 2022. PMID: 35348731 Free PMC article.
-
Dopaminergic System in Promoting Recovery from General Anesthesia.Brain Sci. 2023 Mar 24;13(4):538. doi: 10.3390/brainsci13040538. Brain Sci. 2023. PMID: 37190503 Free PMC article. Review.
-
Ventral pallidum DRD3 potentiates a pallido-habenular circuit driving accumbal dopamine release and cocaine seeking.Neuron. 2021 Jul 7;109(13):2165-2182.e10. doi: 10.1016/j.neuron.2021.05.002. Epub 2021 May 27. Neuron. 2021. PMID: 34048697 Free PMC article.
-
The role of enkephalinergic systems in substance use disorders.Front Syst Neurosci. 2022 Aug 5;16:932546. doi: 10.3389/fnsys.2022.932546. eCollection 2022. Front Syst Neurosci. 2022. PMID: 35993087 Free PMC article. Review.
References
-
- Becchetti A., Gullo F., Bruno G., Dossi E., Lecchi M., Wanke E. (2012). Exact distinction of excitatory and inhibitory neurons in neural networks: a study with GFP-GAD67 neurons optically and electrophysiologically recognized on multielectrode arrays. Front. Neural Circuits 6:63. 10.3389/fncir.2012.00063 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

