Costorage of High Molecular Weight Neurotransmitters in Large Dense Core Vesicles of Mammalian Neurons
- PMID: 30186121
- PMCID: PMC6110924
- DOI: 10.3389/fncel.2018.00272
Costorage of High Molecular Weight Neurotransmitters in Large Dense Core Vesicles of Mammalian Neurons
Abstract
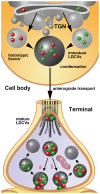
It is today widely accepted that several types of high molecular weight (MW) neurotransmitters produced by neurons are synthesized at the cell body, selectively stored within large dense core vesicles (LDCVs) and anterogradely transported to terminals where they elicit their biological role(s). Among these molecules there are neuropeptides and neurotrophic factors, the main focus of this perspective article. I here first provide a brief resume of the state of art on neuronal secretion, with primary emphasis on the molecular composition and mechanism(s) of filling and release of LDCVs. Then, I discuss the perspectives and future directions of research in the field as regarding the synthesis and storage of multiple high MW transmitters in LDCVs and the possibility that a selective sorting of LDCVs occurs along different neuronal processes and/or their branches. I also consider the ongoing discussion that diverse types of neurons may contain LDCVs with different sets of integral proteins or dial in a different fashion with LDCVs containing the same cargo. In addition, I provide original data on the size of LDCVs in rat dorsal root ganglion neurons and their central terminals in the spinal cord after immunogold labeling for calcitonin gene-related peptide (CGRP), neuropeptide K, substance P, neurokinin A or somatostatin. These data corroborate the idea that, similarly to endocrine cells, LDCVs undergo a process of maturation which involves a homotypic fusion followed by a reduction in size and condensation of cargo. They also give support to the conjecture that release at terminals occurs by cavicapture, a process of partial fusion of the vesicle with the axolemma, accompanied by depletion of cargo and diminution of size.
Keywords: co-localization; co-storage; coexistence; large granular vesicles; neuropeptide; neurotransmission; release; small synaptic vesicles.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

