Intersectional Strategies for Targeting Amacrine and Ganglion Cell Types in the Mouse Retina
- PMID: 30186122
- PMCID: PMC6113359
- DOI: 10.3389/fncir.2018.00066
Intersectional Strategies for Targeting Amacrine and Ganglion Cell Types in the Mouse Retina
Abstract
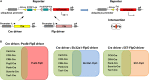
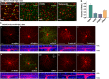
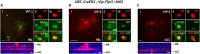
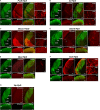
The mammalian retina harbors over 100 different cell types. To understand how retinal circuits work, it is essential to systematically access each type. A widely used approach for achieving targeted transgene expression exploits promoter-driven Cre lines. However, Cre expression in a given transgenic line in the retina and elsewhere in the brain is rarely confined to a single cell type, contributing ambiguity to the interpretation of results from broadly applied manipulations. To obtain unambiguous information about retinal processing, it is desirable to have strategies for further restricting transgene expression to a few or even to a single cell type. We employed an intersectional strategy based on a Cre/Flp double recombinase system to target amacrine and ganglion cell types in the inner retina. We analyzed expression patterns in seven Flp drivers and then created combinational mouse lines by selective cross breeding with Cre drivers. Breeding with Flp drivers can routinely remove labeling from more than 90% of the cells in Cre drivers, leading to only a handful cell types, typically 2-3, remaining in the intersection. Cre/Flp combinatorial mouse lines enabled us to identify and anatomically characterize retinal cell types with greater ease and demonstrated the feasibility of intersectional strategies in retinal research. In addition to the retina, we examined Flp expression in the lateral geniculate nucleus and superior colliculus. Our results establish a foundation for future application of intersectional strategies in the retina and retino-recipient regions.
Keywords: Cre; Flp; amacrine cell; ganglion cell; intersection; retina.
Figures



Similar articles
-
Diverse Central Projection Patterns of Retinal Ganglion Cells.Cell Rep. 2017 Feb 21;18(8):2058-2072. doi: 10.1016/j.celrep.2017.01.075. Cell Rep. 2017. PMID: 28228269 Free PMC article.
-
Genetically targeted binary labeling of retinal neurons.J Neurosci. 2014 Jun 4;34(23):7845-61. doi: 10.1523/JNEUROSCI.2960-13.2014. J Neurosci. 2014. PMID: 24899708 Free PMC article.
-
Pou4f2 knock-in Cre mouse: A multifaceted genetic tool for vision researchers.Mol Vis. 2016 Jun 23;22:705-17. eCollection 2016. Mol Vis. 2016. PMID: 27390513 Free PMC article.
-
Cell-type specification in the retina: Recent discoveries from transcriptomic approaches.Curr Opin Neurobiol. 2023 Aug;81:102752. doi: 10.1016/j.conb.2023.102752. Epub 2023 Jul 25. Curr Opin Neurobiol. 2023. PMID: 37499619 Review.
-
Species Differences in the Nutrition of Retinal Ganglion Cells among Mammals Frequently Used as Animal Models.Cells. 2019 Oct 14;8(10):1254. doi: 10.3390/cells8101254. Cells. 2019. PMID: 31615137 Free PMC article. Review.
Cited by
-
A synaptic temperature sensor for body cooling.Neuron. 2021 Oct 20;109(20):3283-3297.e11. doi: 10.1016/j.neuron.2021.10.001. Neuron. 2021. PMID: 34672983 Free PMC article.
-
Mouse Lines with Cre-Mediated Recombination in Retinal Amacrine Cells.eNeuro. 2022 Feb 14;9(1):ENEURO.0255-21.2021. doi: 10.1523/ENEURO.0255-21.2021. Print 2022 Jan-Feb. eNeuro. 2022. PMID: 35045975 Free PMC article.
-
Functional convergence of on-off direction-selective ganglion cells in the visual thalamus.Curr Biol. 2022 Jul 25;32(14):3110-3120.e6. doi: 10.1016/j.cub.2022.06.023. Epub 2022 Jul 5. Curr Biol. 2022. PMID: 35793680 Free PMC article.
-
Retinal microvascular and neuronal pathologies probed in vivo by adaptive optical two-photon fluorescence microscopy.Elife. 2023 Apr 11;12:e84853. doi: 10.7554/eLife.84853. Elife. 2023. PMID: 37039777 Free PMC article.
-
Retinal ganglion cell interactions shape the developing mammalian visual system.Development. 2020 Dec 7;147(23):dev196535. doi: 10.1242/dev.196535. Development. 2020. PMID: 33288502 Free PMC article. Review.
References
-
- Bassett E. A., Pontoriero G. F., Feng W., Marquardt T., Fini M. E., Williams T., et al. (2007). Conditional deletion of activating protein 2alpha (AP-2alpha) in the developing retina demonstrates non-cell-autonomous roles for AP-2alpha in optic cup development. Mol. Cell. Biol. 27 7497–7510. 10.1128/MCB.00687-07 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

