Distinct Occurrence of Proarrhythmic Afterdepolarizations in Atrial Versus Ventricular Cardiomyocytes: Implications for Translational Research on Atrial Arrhythmia
- PMID: 30186171
- PMCID: PMC6111493
- DOI: 10.3389/fphar.2018.00933
Distinct Occurrence of Proarrhythmic Afterdepolarizations in Atrial Versus Ventricular Cardiomyocytes: Implications for Translational Research on Atrial Arrhythmia
Abstract
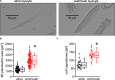
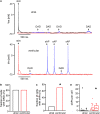
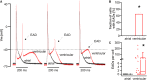
Background: Principal mechanisms of arrhythmia have been derived from ventricular but not atrial cardiomyocytes of animal models despite higher prevalence of atrial arrhythmia (e.g., atrial fibrillation). Due to significant ultrastructural and functional differences, a simple transfer of ventricular proneness toward arrhythmia to atrial arrhythmia is critical. The use of murine models in arrhythmia research is widespread, despite known translational limitations. We here directly compare atrial and ventricular mechanisms of arrhythmia to identify critical differences that should be considered in murine models for development of antiarrhythmic strategies for atrial arrhythmia. Methods and Results: Isolated murine atrial and ventricular myocytes were analyzed by wide field microscopy and subjected to a proarrhythmic protocol during patch-clamp experiments. As expected, the spindle shaped atrial myocytes showed decreased cell area and membrane capacitance compared to the rectangular shaped ventricular myocytes. Though delayed afterdepolarizations (DADs) could be evoked in a similar fraction of both cell types (80% of cells each), these led significantly more often to the occurrence of spontaneous action potentials (sAPs) in ventricular myocytes. Interestingly, numerous early afterdepolarizations (EADs) were observed in the majority of ventricular myocytes, but there was no EAD in any atrial myocyte (EADs per cell; atrial myocytes: 0 ± 0; n = 25/12 animals; ventricular myocytes: 1.5 [0-43]; n = 20/12 animals; p < 0.05). At the same time, the action potential duration to 90% decay (APD90) was unaltered and the APD50 even increased in atrial versus ventricular myocytes. However, the depolarizing L-type Ca2+ current (ICa) and Na+/Ca2+-exchanger inward current (INCX) were significantly smaller in atrial versus ventricular myocytes. Conclusion: In mice, atrial myocytes exhibit a substantially distinct occurrence of proarrhythmic afterdepolarizations compared to ventricular myocytes, since they are in a similar manner susceptible to DADs but interestingly seem to be protected against EADs and show less sAPs. Key factors in the generation of EADs like ICa and INCX were significantly reduced in atrial versus ventricular myocytes, which may offer a mechanistic explanation for the observed protection against EADs. These findings may be of relevance for current studies on atrial level in murine models to develop targeted strategies for the treatment of atrial arrhythmia.
Keywords: afterdepolarizations; atrial arrhythmia; atrial cardiomyocytes; atrioventricular differences; translational research.
Figures



Similar articles
-
Hypokalemia Promotes Arrhythmia by Distinct Mechanisms in Atrial and Ventricular Myocytes.Circ Res. 2020 Mar 27;126(7):889-906. doi: 10.1161/CIRCRESAHA.119.315641. Epub 2020 Feb 19. Circ Res. 2020. PMID: 32070187 Free PMC article.
-
Proarrhythmia in a non-failing murine model of cardiac-specific Na+/Ca 2+ exchanger overexpression: whole heart and cellular mechanisms.Basic Res Cardiol. 2012 Mar;107(2):247. doi: 10.1007/s00395-012-0247-7. Epub 2012 Feb 11. Basic Res Cardiol. 2012. PMID: 22327339 Free PMC article.
-
Tolterodine reduces veratridine-augmented late INa, reverse-INCX and early afterdepolarizations in isolated rabbit ventricular myocytes.Acta Pharmacol Sin. 2016 Nov;37(11):1432-1441. doi: 10.1038/aps.2016.76. Epub 2016 Aug 29. Acta Pharmacol Sin. 2016. PMID: 27569391 Free PMC article.
-
Cardiomyocytes hypertrophic status after myocardial infarction determines distinct types of arrhythmia: role of the ryanodine receptor.Prog Biophys Mol Biol. 2010 Sep;103(1):71-80. doi: 10.1016/j.pbiomolbio.2010.01.002. Epub 2010 Jan 28. Prog Biophys Mol Biol. 2010. PMID: 20109482 Review.
-
Afterdepolarizations and triggered activity as a mechanism for clinical arrhythmias.Pacing Clin Electrophysiol. 2018 Jun 19. doi: 10.1111/pace.13419. Online ahead of print. Pacing Clin Electrophysiol. 2018. PMID: 29920724 Review.
Cited by
-
Higher Na+-Ca2+ Exchanger Function and Triggered Activity Contribute to Male Predisposition to Atrial Fibrillation.Int J Mol Sci. 2022 Sep 14;23(18):10724. doi: 10.3390/ijms231810724. Int J Mol Sci. 2022. PMID: 36142639 Free PMC article.
-
A comparative review on heart ion channels, action potentials and electrocardiogram in rodents and human: extrapolation of experimental insights to clinic.Lab Anim Res. 2021 Sep 8;37(1):25. doi: 10.1186/s42826-021-00102-3. Lab Anim Res. 2021. PMID: 34496976 Free PMC article. Review.
-
Development of a robust induced pluripotent stem cell atrial cardiomyocyte differentiation protocol to model atrial arrhythmia.Stem Cell Res Ther. 2023 Jul 27;14(1):183. doi: 10.1186/s13287-023-03405-5. Stem Cell Res Ther. 2023. PMID: 37501071 Free PMC article.
-
Cre recombinase affects calcium dynamics already in young mice.Front Pharmacol. 2025 Mar 26;16:1558573. doi: 10.3389/fphar.2025.1558573. eCollection 2025. Front Pharmacol. 2025. PMID: 40206076 Free PMC article.
-
HFpEF and Atrial Fibrillation: The Enigmatic Interplay of Dysmetabolism, Biomarkers, and Vascular Endothelial Dysfunction.Dis Markers. 2022 Oct 25;2022:9539676. doi: 10.1155/2022/9539676. eCollection 2022. Dis Markers. 2022. PMID: 36330203 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

