Estrone-3-Sulfate Stimulates the Proliferation of T47D Breast Cancer Cells Stably Transfected With the Sodium-Dependent Organic Anion Transporter SOAT (SLC10A6)
- PMID: 30186172
- PMCID: PMC6111516
- DOI: 10.3389/fphar.2018.00941
Estrone-3-Sulfate Stimulates the Proliferation of T47D Breast Cancer Cells Stably Transfected With the Sodium-Dependent Organic Anion Transporter SOAT (SLC10A6)
Abstract
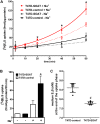
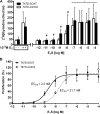
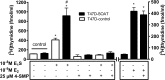
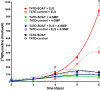
Estrogens play a pivotal role in the development and proliferation of hormone-dependent breast cancer. Apart from free estrogens, which can directly activate the estrogen receptor (ER) of tumor cells, sulfo-conjugated steroids, which maintain high plasma concentrations even after menopause, first have to be imported into tumor cells by carrier-mediated uptake and then can be cleaved by the steroid sulfatase to finally activate ERs and cell proliferation. In the present study, expression of the sodium-dependent organic anion transporter SOAT was analyzed in breast cancer and its role for hormone-dependent proliferation of T47D breast cancer cells was elucidated. The SOAT protein was localized to the ductal epithelium of the mammary gland by immunohistochemistry. SOAT showed high expression in different pathologies of the breast with a clear ductal localization, including ductal hyperplasia, intraductal papilloma, and intraductal carcinoma. In a larger breast cancer cDNA array, SOAT mRNA expression was high in almost all adenocarcinoma specimen, but expression did not correlate with either the ER, progesterone receptor, or human epidermal growth factor receptor 2 status. Furthermore, SOAT expression did not correlate with tumor stage or grade, indicating widespread SOAT expression in breast cancer. To analyze the role of SOAT for breast cancer cell proliferation, T47D cells were stably transfected with SOAT and incubated under increasing concentrations of estrone-3-sulfate (E1S) and estradiol at physiologically relevant concentrations. Cell proliferation was significantly increased by 10-9 M estradiol as well as by E1S with EC50 of 2.2 nM. In contrast, T47D control cells showed 10-fold lower sensitivity to E1S stimulation with EC50 of 21.7 nM. The E1S-stimulated proliferation of SOAT-T47D cells was blocked by the SOAT inhibitor 4-sulfooxymethylpyrene.
In conclusion: The present study clearly demonstrates expression of SOAT in breast cancer tissue with ductal localization. SOAT inhibition can block the E1S-stimulated proliferation of T47D breast cancer cells, demonstrating that SOAT is an interesting novel drug target from the group of E1S uptake carriers for anti-proliferative breast cancer therapy.
Keywords: SLC10A6; SOAT; T47D; breast cancer; estrone-3-sulfate; proliferation; sulfate steroid; transport.
Figures


Similar articles
-
Role of the Sodium-Dependent Organic Anion Transporter (SOAT/SLC10A6) in Physiology and Pathophysiology.Int J Mol Sci. 2023 Jun 8;24(12):9926. doi: 10.3390/ijms24129926. Int J Mol Sci. 2023. PMID: 37373074 Free PMC article. Review.
-
Rare genetic variants in the sodium-dependent organic anion transporter SOAT (SLC10A6): Effects on transport function and membrane expression.J Steroid Biochem Mol Biol. 2018 May;179:26-35. doi: 10.1016/j.jsbmb.2017.09.004. Epub 2017 Sep 8. J Steroid Biochem Mol Biol. 2018. PMID: 28893621
-
Sodium-dependent organic anion transporter (Slc10a6-/-) knockout mice show normal spermatogenesis and reproduction, but elevated serum levels for cholesterol sulfate.J Steroid Biochem Mol Biol. 2018 May;179:45-54. doi: 10.1016/j.jsbmb.2017.07.019. Epub 2017 Jul 22. J Steroid Biochem Mol Biol. 2018. PMID: 28743544
-
Transport of steroid 3-sulfates and steroid 17-sulfates by the sodium-dependent organic anion transporter SOAT (SLC10A6).J Steroid Biochem Mol Biol. 2018 May;179:20-25. doi: 10.1016/j.jsbmb.2017.09.013. Epub 2017 Sep 23. J Steroid Biochem Mol Biol. 2018. PMID: 28951227
-
Importance of estrogen sulfates in breast cancer.J Steroid Biochem. 1989;34(1-6):155-63. doi: 10.1016/0022-4731(89)90077-0. J Steroid Biochem. 1989. PMID: 2560511 Review.
Cited by
-
SOAT1 methylation is associated with coronary heart disease.Lipids Health Dis. 2019 Nov 4;18(1):192. doi: 10.1186/s12944-019-1138-9. Lipids Health Dis. 2019. PMID: 31684966 Free PMC article.
-
Role of the Sodium-Dependent Organic Anion Transporter (SOAT/SLC10A6) in Physiology and Pathophysiology.Int J Mol Sci. 2023 Jun 8;24(12):9926. doi: 10.3390/ijms24129926. Int J Mol Sci. 2023. PMID: 37373074 Free PMC article. Review.
-
Identification of epigenetic dysregulation gene markers and immune landscape in kidney renal clear cell carcinoma by comprehensive genomic analysis.Front Immunol. 2022 Aug 18;13:901662. doi: 10.3389/fimmu.2022.901662. eCollection 2022. Front Immunol. 2022. PMID: 36059531 Free PMC article.
-
SLC10A7, an orphan member of the SLC10 family involved in congenital disorders of glycosylation.Hum Genet. 2022 Jul;141(7):1287-1298. doi: 10.1007/s00439-021-02420-x. Epub 2022 Jan 8. Hum Genet. 2022. PMID: 34999954
-
Substrate Specificities and Inhibition Pattern of the Solute Carrier Family 10 Members NTCP, ASBT and SOAT.Front Mol Biosci. 2021 May 17;8:689757. doi: 10.3389/fmolb.2021.689757. eCollection 2021. Front Mol Biosci. 2021. PMID: 34079822 Free PMC article.
References
-
- Adams J. B., Wong M. S. F. (1968). Paraendocrine behaviour of human breast carcinoma: in vitro transformation of steroids to physiologically active hormones. J. Endocr. 41 41–52. 10.1677/joe.0.0410041 - DOI
-
- American Joint Committee on Cancer (2002). in Cancer Staging Manual, 6th Edn, eds Greene F. L., Page D. L., Fleming I. D., Fritz A. G., Balch C. M., Haller D. G., et al. (New York, NY: Springer-Verlag; ). 10.1007/978-1-4757-3656-4 - DOI
-
- Bakhaus K., Bennien J., Fietz D., Sánchez-Guijo A., Hartmann M., Serafini R., et al. (2018). Sodium-dependent organic anion transporter (Slc10a6-/-) knockout mice show normal spermatogenesis and reproduction, but elevated serum levels for cholesterol sulfate. J. Steroid Biochem. Mol. Biol. 179 45–54. 10.1016/j.jsbmb.2017.07.019 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

