Emodin Attenuates Lipopolysaccharide-Induced Acute Liver Injury via Inhibiting the TLR4 Signaling Pathway in vitro and in vivo
- PMID: 30186181
- PMCID: PMC6113398
- DOI: 10.3389/fphar.2018.00962
Emodin Attenuates Lipopolysaccharide-Induced Acute Liver Injury via Inhibiting the TLR4 Signaling Pathway in vitro and in vivo
Abstract
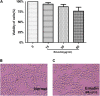
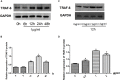
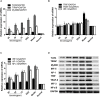
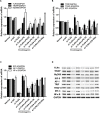
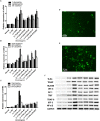
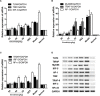
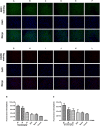
Aims: Emodin is an anthraquinone with potential anti-inflammatory properties. However, the possible molecular mechanisms and protective effects of emodin are not clear. The objective of this study was to investigate the possible molecular mechanisms and protective effects of emodin on lipopolysaccharide (LPS)-induced acute liver injury (ALI) via the Toll-like receptor 4 (TLR4) signaling pathway in the Raw264.7 cell line and in Balb/c mice. Methods: This study established an inflammatory cellular model and induced an ALI animal model. TLR4 was overexpressed by lentivirus and downregulated by small interfering RNA (siRNA) technology. The mRNA and protein levels of TLR4 and downstream molecules were detected in cells and liver tissue. The tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 levels in supernatant and serum were determined by ELISA. The distribution and expression of mannose receptor C type 1 (CD206) and arginase 1 (ARG1) in the liver were tested by immunofluorescence. Mouse liver function and histopathological observations were assessed. Results: Administration of emodin reduced the protein and/or mRNA levels of TLR4 and its downstream molecules following LPS challenge in Raw264.7 cells and in an animal model. Additionally, emodin suppressed the expression of TNF-α and IL-6 in cell culture supernatant and serum. The inhibitory effect of emodin was also confirmed in RAW264.7 cells, in which TLR4 was overexpressed or knocked down. Additionally, ARG1 and CD206 were elevated in the emodin groups. Emodin also decreased serum ALT and AST levels and alleviated the liver histopathological damage induced by LPS. Conclusion: Emodin showed excellent hepatoprotective effects against LPS-induced ALI, possibly by inhibiting TLR4 signaling pathways.
Keywords: TLR4; acute liver injury; emodin; lipopolysaccharide; signaling pathway.
Figures
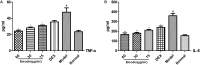


Similar articles
-
Attenuation of Inflammation by Emodin in Lipopolysaccharide-induced Acute Kidney Injury via Inhibition of Toll-like Receptor 2 Signal Pathway.Iran J Kidney Dis. 2015 May;9(3):202-8. Iran J Kidney Dis. 2015. PMID: 25957424
-
Emodin Protects Against Lipopolysaccharide-Induced Acute Lung Injury via the JNK/Nur77/c-Jun Signaling Pathway.Front Pharmacol. 2022 Mar 17;13:717271. doi: 10.3389/fphar.2022.717271. eCollection 2022. Front Pharmacol. 2022. PMID: 35370650 Free PMC article.
-
Emodin Protects Sepsis Associated Damage to the Intestinal Mucosal Barrier Through the VDR/ Nrf2 /HO-1 Pathway.Front Pharmacol. 2021 Dec 20;12:724511. doi: 10.3389/fphar.2021.724511. eCollection 2021. Front Pharmacol. 2021. PMID: 34987380 Free PMC article.
-
Zerumbone Protects against Carbon Tetrachloride (CCl4)-Induced Acute Liver Injury in Mice via Inhibiting Oxidative Stress and the Inflammatory Response: Involving the TLR4/NF-κB/COX-2 Pathway.Molecules. 2019 May 22;24(10):1964. doi: 10.3390/molecules24101964. Molecules. 2019. PMID: 31121820 Free PMC article.
-
Advances in the mechanism of emodin-induced hepatotoxicity.Heliyon. 2024 Jun 25;10(13):e33631. doi: 10.1016/j.heliyon.2024.e33631. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39027614 Free PMC article. Review.
Cited by
-
Systems pharmacology dissection of the mechanisms and therapeutic potential of Cassiae semen for hepatoprotection and brightening eyes.J Food Drug Anal. 2022 Sep 15;30(3):417-426. doi: 10.38212/2224-6614.3417. J Food Drug Anal. 2022. PMID: 39666297 Free PMC article.
-
Potential of Plant-Derived Compounds in Preventing and Reversing Organ Fibrosis and the Underlying Mechanisms.Cells. 2024 Feb 28;13(5):421. doi: 10.3390/cells13050421. Cells. 2024. PMID: 38474385 Free PMC article. Review.
-
Hepatoprotective Effect of Kaempferol-A Review.Molecules. 2025 Apr 25;30(9):1913. doi: 10.3390/molecules30091913. Molecules. 2025. PMID: 40363718 Free PMC article. Review.
-
Emodin Alleviates Severe Acute Pancreatitis-Associated Acute Lung Injury by Inhibiting the Cold-Inducible RNA-Binding Protein (CIRP)-Mediated Activation of the NLRP3/IL-1β/CXCL1 Signaling.Front Pharmacol. 2021 Apr 23;12:655372. doi: 10.3389/fphar.2021.655372. eCollection 2021. Front Pharmacol. 2021. PMID: 33967799 Free PMC article.
-
Aloin Preconditioning Attenuates Hepatic Ischemia/Reperfusion Injury via Inhibiting TLR4/MyD88/NF-κB Signal Pathway In Vivo and In Vitro.Oxid Med Cell Longev. 2019 Nov 20;2019:3765898. doi: 10.1155/2019/3765898. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31827674 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

