Metabolic Profiles Associated With Metformin Efficacy in Cancer
- PMID: 30186229
- PMCID: PMC6110930
- DOI: 10.3389/fendo.2018.00372
Metabolic Profiles Associated With Metformin Efficacy in Cancer
Abstract
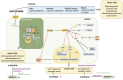
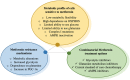
Metformin is one of the most commonly prescribed medications for the treatment of type 2 diabetes. Numerous reports have suggested potential anti-cancerous and cancer preventive properties of metformin, although these findings vary depending on the intrinsic properties of the tumor, as well as the systemic physiology of patients. These intriguing studies have led to a renewed interest in metformin use in the oncology setting, and fueled research to unveil its elusive mode of action. It is now appreciated that metformin inhibits complex I of the electron transport chain in mitochondria, causing bioenergetic stress in cancer cells, and rendering them dependent on glycolysis for ATP production. Understanding the mode of action of metformin and the consequences of its use on cancer cell bioenergetics permits the identification of cancer types most susceptible to metformin action. Such knowledge may also shed light on the varying results to metformin usage that have been observed in clinical trials. In this review, we discuss metabolic profiles of cancer cells that are associated with metformin sensitivity, and rationalize combinatorial treatment options. We use the concept of bioenergetic flexibility, which has recently emerged in the field of cancer cell metabolism, to further understand metabolic rearrangements that occur upon metformin treatment. Finally, we advance the notion that metabolic fitness of cancer cells increases during progression to metastatic disease and the emergence of therapeutic resistance. As a result, sophisticated combinatorial approaches that prevent metabolic compensatory mechanisms will be required to effectively manage metastatic disease.
Keywords: breast cancer; cancer; diabetes; metabolism; metformin; mitochondria; mitochondrial drugs; phenformin.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials