Methods for Lipid Droplet Biophysical Characterization in Flaviviridae Infections
- PMID: 30186265
- PMCID: PMC6110928
- DOI: 10.3389/fmicb.2018.01951
Methods for Lipid Droplet Biophysical Characterization in Flaviviridae Infections
Abstract
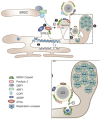
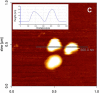
Lipid droplets (LDs) are intracellular organelles for neutral lipid storage, originated from the endoplasmic reticulum. They play an essential role in lipid metabolism and cellular homeostasis. In fact, LDs are complex organelles, involved in many more cellular processes than those initially proposed. They have been extensively studied in the context of LD-associated pathologies. In particular, LDs have emerged as critical for virus replication and assembly. Viruses from the Flaviviridae family, namely dengue virus (DENV), hepatitis C virus (HCV), West Nile virus (WNV), and Zika virus (ZIKV), interact with LDs to usurp the host lipid metabolism for their own viral replication and pathogenesis. In general, during Flaviviridae infections it is observed an increasing number of host intracellular LDs. Several viral proteins interact with LDs during different steps of the viral life cycle. The HCV core protein and DENV capsid protein, extensively interact with LDs to regulate their replication and assembly. Detailed studies of LDs in viral infections may contribute for the development of possible inhibitors of key steps of viral replication. Here, we reviewed different techniques that can be used to characterize LDs isolated from infected or non-infected cells. Microscopy studies have been commonly used to observe LDs accumulation and localization in infected cell cultures. Fluorescent dyes, which may affect LDs directly, are widely used to probe LDs but there are also approaches that do not require the use of fluorescence, namely stimulated Raman scattering, electron and atomic force microscopy-based approaches. These three are powerful techniques to characterize LDs morphology. Raman scattering microscopy allows studying LDs in a single cell. Electron and atomic force microscopies enable a better characterization of LDs in terms of structure and interaction with other organelles. Other biophysical techniques, such as dynamic light scattering and zeta potential are also excellent to characterize LDs in terms of size in a simple and fast way and test possible LDs interaction with viral proteins. These methodologies are reviewed in detail, in the context of viral studies.
Keywords: Flaviviridae; LDs-associated proteins; light scattering; lipid droplet; microscopy; viral proteins.
Figures

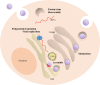





 ) and the nucleus (N) can be identified. The scale bar corresponds to 1 μm. Most hepatocytes of the infected animal contain multiple small (ca. 2 μm diameter) LDs. They may be indicative of ongoing viral replication [reprinted from Martin et al. (2003). Copyright (2003) National Academy of Sciences, United States].
) and the nucleus (N) can be identified. The scale bar corresponds to 1 μm. Most hepatocytes of the infected animal contain multiple small (ca. 2 μm diameter) LDs. They may be indicative of ongoing viral replication [reprinted from Martin et al. (2003). Copyright (2003) National Academy of Sciences, United States].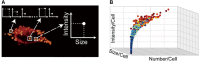

Similar articles
-
Modulation of Lipid Droplet Metabolism-A Potential Target for Therapeutic Intervention in Flaviviridae Infections.Front Microbiol. 2017 Nov 28;8:2286. doi: 10.3389/fmicb.2017.02286. eCollection 2017. Front Microbiol. 2017. PMID: 29234310 Free PMC article. Review.
-
Hijacking of Lipid Droplets by Hepatitis C, Dengue and Zika Viruses-From Viral Protein Moonlighting to Extracellular Release.Int J Mol Sci. 2020 Oct 24;21(21):7901. doi: 10.3390/ijms21217901. Int J Mol Sci. 2020. PMID: 33114346 Free PMC article. Review.
-
West Nile Virus Capsid Protein Interacts With Biologically Relevant Host Lipid Systems.Front Cell Infect Microbiol. 2019 Feb 6;9:8. doi: 10.3389/fcimb.2019.00008. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30788291 Free PMC article.
-
Dengue virus capsid protein interacts specifically with very low-density lipoproteins.Nanomedicine. 2014 Jan;10(1):247-55. doi: 10.1016/j.nano.2013.06.004. Epub 2013 Jun 20. Nanomedicine. 2014. PMID: 23792329
-
Lipid Droplets and Their Participation in Zika Virus Infection.Int J Mol Sci. 2022 Oct 20;23(20):12584. doi: 10.3390/ijms232012584. Int J Mol Sci. 2022. PMID: 36293437 Free PMC article. Review.
Cited by
-
Dengue and Zika Viruses: Epidemiological History, Potential Therapies, and Promising Vaccines.Trop Med Infect Dis. 2020 Sep 23;5(4):150. doi: 10.3390/tropicalmed5040150. Trop Med Infect Dis. 2020. PMID: 32977703 Free PMC article. Review.
-
Molecular Events Occurring in Lipophagy and Its Regulation in Flaviviridae Infection.Front Microbiol. 2021 May 21;12:651952. doi: 10.3389/fmicb.2021.651952. eCollection 2021. Front Microbiol. 2021. PMID: 34093468 Free PMC article. Review.
-
Metabolic alterations upon SARS-CoV-2 infection and potential therapeutic targets against coronavirus infection.Signal Transduct Target Ther. 2023 Jun 7;8(1):237. doi: 10.1038/s41392-023-01510-8. Signal Transduct Target Ther. 2023. PMID: 37286535 Free PMC article. Review.
-
Label-free third harmonic generation imaging and quantification of lipid droplets in live filamentous fungi.Sci Rep. 2022 Nov 5;12(1):18760. doi: 10.1038/s41598-022-23502-4. Sci Rep. 2022. PMID: 36335164 Free PMC article.
-
Finding intracellular lipid droplets from the single-cell biolens' signature in a holographic flow-cytometry assay.Biomed Opt Express. 2022 Oct 4;13(11):5585-5598. doi: 10.1364/BOE.460204. eCollection 2022 Nov 1. Biomed Opt Express. 2022. PMID: 36733743 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

