Impact of autologous whole blood administration upon experimental mouse models of acute Trypanosoma cruzi infection
- PMID: 30186314
- PMCID: PMC6117903
- DOI: 10.1186/s40409-018-0157-8
Impact of autologous whole blood administration upon experimental mouse models of acute Trypanosoma cruzi infection
Abstract
Background: Autologous whole blood (AWB) administration is described as alternative/complementary medical practice widely employed in medical and veterinary therapy against infections, chronic pathologies and neoplasias. Our aim is to investigate in vivo biological effect of AWB using healthy murine models under the course of Trypanosoma cruzi acute infection.
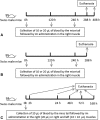
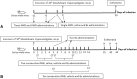
Methods: The first set of studies consisted of injecting different volumes of AWB and saline (SAL) into the posterior region of quadriceps muscle of healthy male Swiss mice under distinct therapeutic schemes evaluating: animal behavior, body and organ weight, hemogram, plasmatic biochemical markers for tissue damage and inflammatory cytokine levels and profile. To assess the impact on the experimental T. cruzi infection, different schemes (prior and post infection) and periods of AWB administration (from one up to 10 days) were conducted, also employing heterologous whole blood (HWB) and evaluating plasma cytokine profile.
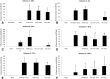
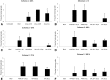
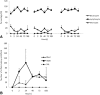
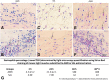
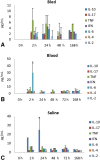
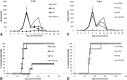
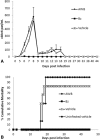
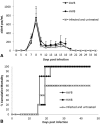
Results: No major adverse events were observed in healthy AWB-treated mice, except gait impairment in animals that received three doses of 20 μL AWB in the same hind limb. AWB and SAL triggered an immediate polymorphonuclear response followed by mononuclear infiltrate. Although SAL triggered an inflammatory response, the kinetics and intensity of the histological profile and humoral mediator levels were different from AWB, the latter occurring earlier and more intensely with concomitant elevation of plasma IL-6. Inflammatory peak response of SAL, mainly composed of mononuclear cells with IL-10, was increased at 24 h. According to the mouse model of acute T. cruzi infection, only minor decreases (< 30%) in the parasitemia levels were produced by AWB and HWB given before and after infection, without protecting against mortality. Rises in IFN-gamma, TNF-alpha and IL-6 were detected at 9 dpi in all infected animals as compared to uninfected mice but only Bz displayed a statistically significant diminution (p = 0.02) in TNF-alpha levels than infected and untreated mice.
Conclusions: This study revealed that the use of autologous whole blood (AWB) in the acute model employed was unable to reduce the parasitic load of infected mice, providing only a minor decrease in parasitemia levels (up to 30%) but without protecting against animal mortality. Further in vivo studies will be necessary to elucidate the effective impact of this procedure.
Keywords: Alternative therapy; Autologous blood; Mouse models; Trypanosoma cruzi.
Conflict of interest statement
Our present study was submitted to Fiocruz Committee of Ethics for the Use of Animals (CEUA) and approved under the license number CEUA L-032/2016.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Thioridazine aggravates skeletal myositis, systemic and liver inflammation in Trypanosoma cruzi-infected and benznidazole-treated mice.Int Immunopharmacol. 2020 Aug;85:106611. doi: 10.1016/j.intimp.2020.106611. Epub 2020 May 21. Int Immunopharmacol. 2020. PMID: 32447223
-
Curcumin Enhances the Anti-Trypanosoma cruzi Activity of Benznidazole-Based Chemotherapy in Acute Experimental Chagas Disease.Antimicrob Agents Chemother. 2016 May 23;60(6):3355-64. doi: 10.1128/AAC.00343-16. Print 2016 Jun. Antimicrob Agents Chemother. 2016. PMID: 27001816 Free PMC article.
-
Could phenothiazine-benznidazole combined chemotherapy be effective in controlling heart parasitism and acute infectious myocarditis?Pharmacol Res. 2020 Aug;158:104907. doi: 10.1016/j.phrs.2020.104907. Epub 2020 May 19. Pharmacol Res. 2020. PMID: 32416214
-
Oral Route Driven Acute Trypanosoma cruzi Infection Unravels an IL-6 Dependent Hemostatic Derangement.Front Immunol. 2019 May 14;10:1073. doi: 10.3389/fimmu.2019.01073. eCollection 2019. Front Immunol. 2019. PMID: 31139194 Free PMC article.
-
Concomitant Benznidazole and Suramin Chemotherapy in Mice Infected with a Virulent Strain of Trypanosoma cruzi.Antimicrob Agents Chemother. 2015 Oct;59(10):5999-6006. doi: 10.1128/AAC.00779-15. Epub 2015 Jul 13. Antimicrob Agents Chemother. 2015. PMID: 26169419 Free PMC article.
Cited by
-
Hippo/YAP signaling's multifaceted crosstalk in cancer.Front Cell Dev Biol. 2025 Jul 2;13:1595362. doi: 10.3389/fcell.2025.1595362. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40673277 Free PMC article. Review.
-
Predicting Blood Parasite Load and Influence of Expression of iNOS on the Effect Size of Clinical Laboratory Parameters in Acute Trypanosoma cruzi Infection With Different Inoculum Concentrations in C57BL/6 Mice.Front Immunol. 2022 Mar 18;13:850037. doi: 10.3389/fimmu.2022.850037. eCollection 2022. Front Immunol. 2022. PMID: 35371021 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

