Preparation and characterization of norcantharidin liposomes modified with stearyl glycyrrhetinate
- PMID: 30186382
- PMCID: PMC6122258
- DOI: 10.3892/etm.2018.6416
Preparation and characterization of norcantharidin liposomes modified with stearyl glycyrrhetinate
Abstract
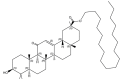
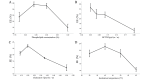
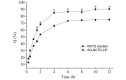
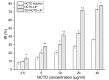
In the current study, norcantharidin (NCTD)-loaded liposomes (LIPs) modified with stearyl glycyrrhetinate (SG; SG-NCTD-LIP) were prepared by the ethanol injection method. To increase the drug encapsulation efficiency (EE), the formulation of NCTD-LIP was optimized by single factor test and orthogonal design. The release of NCTD in vitro from SG-NCTD-LIP was evaluated by equilibrium dialysis. The cytotoxicity of SG-NCTD-LIP in HepG2 was investigated by MTT assay. The results revealed that the EE of liposomes was ~27.80%, the average SG-NCTD-LIP was 87.5 nm, the in vitro NCTD release from SG-NCTD-LIP was delayed compared with NCTD in solution and the drug-release kinetic followed a first-order model. MTT assays revealed increased cytotoxicity activity against HepG2 cells for SG-NCTD-LIP compared with free NCTD. In conclusion, SG-NCTD-LIP prepared in the present study may be a promising liposomal drug delivery system for anticancer drugs in liver-targeting therapy.
Keywords: drug delivery system; liposomes; liver-targeting; norcantharidin; stearyl glycyrrhetinate.
Figures

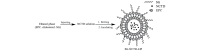

Similar articles
-
Folate receptor-targeted liposomes loaded with a diacid metabolite of norcantharidin enhance antitumor potency for H22 hepatocellular carcinoma both in vitro and in vivo.Int J Nanomedicine. 2016 Apr 4;11:1395-412. doi: 10.2147/IJN.S96862. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27110110 Free PMC article.
-
Preparation, characterization and anticancer activity of norcantharidin-loaded poly(ethylene glycol)-poly(caprolactone) amphiphilic block copolymer micelles.Pharmazie. 2012 Sep;67(9):781-8. Pharmazie. 2012. PMID: 23016451
-
Liposomal Nanodrug Based on Norcantharidin Derivative for Increased in Vivo Activity.AAPS PharmSciTech. 2023 May 10;24(5):118. doi: 10.1208/s12249-023-02572-1. AAPS PharmSciTech. 2023. PMID: 37165275
-
Preparation and evaluation of norcantharidin-encapsulated liposomes modified with a novel CD19 monoclonal antibody 2E8.J Huazhong Univ Sci Technolog Med Sci. 2010 Apr;30(2):240-7. doi: 10.1007/s11596-010-0222-1. Epub 2010 Apr 21. J Huazhong Univ Sci Technolog Med Sci. 2010. PMID: 20407882
-
Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities.Chin Med. 2020 May 29;15:55. doi: 10.1186/s13020-020-00338-6. eCollection 2020. Chin Med. 2020. PMID: 32514288 Free PMC article. Review.
Cited by
-
Establishment and evaluation of glucose-modified nanocomposite liposomes for the treatment of cerebral malaria.J Nanobiotechnology. 2022 Jul 6;20(1):318. doi: 10.1186/s12951-022-01493-8. J Nanobiotechnology. 2022. PMID: 35794597 Free PMC article.
-
In vitro and in vivo Evaluation of a Novel Estrogen-Targeted PEGylated Oxaliplatin Liposome for Gastric Cancer.Int J Nanomedicine. 2021 Dec 23;16:8279-8303. doi: 10.2147/IJN.S340180. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34992365 Free PMC article.
-
Preparation and in vitro and in vivo evaluations of 10-hydroxycamptothecin liposomes modified with stearyl glycyrrhetinate.Drug Deliv. 2019 Dec;26(1):673-679. doi: 10.1080/10717544.2019.1636422. Drug Deliv. 2019. PMID: 31266376 Free PMC article.
-
Review targeted drug delivery systems for norcantharidin in cancer therapy.J Nanobiotechnology. 2022 Dec 3;20(1):509. doi: 10.1186/s12951-022-01703-3. J Nanobiotechnology. 2022. PMID: 36463199 Free PMC article. Review.
-
A new core-shell-type nanoparticle loaded with paclitaxel/norcantharidin and modified with APRPG enhances anti-tumor effects in hepatocellular carcinoma.Front Oncol. 2022 Sep 14;12:932156. doi: 10.3389/fonc.2022.932156. eCollection 2022. Front Oncol. 2022. PMID: 36185205 Free PMC article.
References
-
- Wei CM, Wang BJ, Ma Y, Sun ZP, Li XL, Guo RC. Pharmacokinetics and biodistributionof 3H-norcantharidin in mice. Yao Xue Xue Bao. 2007;42:516–519. (In Chinese) - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
