MicroRNA-101-3p inhibits proliferation in retinoblastoma cells by targeting EZH2 and HDAC9
- PMID: 30186385
- PMCID: PMC6122260
- DOI: 10.3892/etm.2018.6405
MicroRNA-101-3p inhibits proliferation in retinoblastoma cells by targeting EZH2 and HDAC9
Abstract
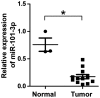
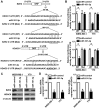
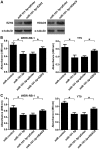
Retinoblastoma is the most frequent intraocular malignant tumor type to occur in childhood. MicroRNA (miR)-101-3p has been reported to function as a tumor suppressor in various types of cancer. However, the biological function and underlying mechanisms of miR-101-3p in retinoblastoma are largely unknown. In the present study, it was identified that miR-101-3p was downregulated in retinoblastoma. MTT and flow cytometry assays demonstrated that ectopic overexpression of miR-101-3p significantly inhibited cell viability and cell cycle progression in WERI-Rb-1 and Y79 cells. In vivo mouse experiments further confirmed the anti-proliferative role of miR-101-3p in retinoblastoma. Additionally, predictions with TargetScan software indicated that the 3'-untranslated regions of enhancer of zeste homolog 2 (EZH2) and histone deacetylase (HDAC9) mRNAs are targeted by miR-101-3p. Accordingly, a dual luciferase reporter gene assay demonstrated that miR-101-3p directly targeted EZH2 and HDAC9 to suppress the proliferation of retinoblastoma cells. Meanwhile, the restoration of EZH2 or HDAC9 expression countered the anti-proliferative effect of miR-101-3p on WERI-Rb-1 and Y79 cells. Collectively, these data highlight the role of miR-101-3p in the tumorigenesis of retinoblastoma, and indicate its suitability as a novel therapeutic target.
Keywords: enhancer of zeste homolog 2; histone deacetylase 9; microRNA-101-3p; proliferation; retinoblastoma.
Figures


Similar articles
-
MicroRNA-361-3p regulates retinoblastoma cell proliferation and stemness by targeting hedgehog signaling.Exp Ther Med. 2019 Feb;17(2):1154-1162. doi: 10.3892/etm.2018.7062. Epub 2018 Dec 6. Exp Ther Med. 2019. PMID: 30679988 Free PMC article.
-
miR-130a-3p Enhances the Chemosensitivity of Y79 Retinoblastoma Cells to Vincristine by Targeting PAX6 Expression.Curr Eye Res. 2022 Mar;47(3):418-425. doi: 10.1080/02713683.2021.1984537. Epub 2021 Sep 30. Curr Eye Res. 2022. PMID: 34547965
-
microRNA -378a-3p Restrains the Proliferation of Retinoblastoma Cells but Promotes Apoptosis of Retinoblastoma Cells via Inhibition of FOXG1.Invest Ophthalmol Vis Sci. 2020 May 11;61(5):31. doi: 10.1167/iovs.61.5.31. Invest Ophthalmol Vis Sci. 2020. PMID: 32428232 Free PMC article.
-
MicroRNA-101 inhibits human hepatocellular carcinoma progression through EZH2 downregulation and increased cytostatic drug sensitivity.J Hepatol. 2014 Mar;60(3):590-8. doi: 10.1016/j.jhep.2013.10.028. Epub 2013 Nov 6. J Hepatol. 2014. PMID: 24211739
-
Downregulation of microRNA-320a inhibits proliferation and induces apoptosis of retinoblastoma cells via targeting TUSC3.Exp Ther Med. 2020 Nov;20(5):9. doi: 10.3892/etm.2020.9137. Epub 2020 Aug 25. Exp Ther Med. 2020. PMID: 32934674 Free PMC article.
Cited by
-
Knockdown of lncRNA HOTTIP Inhibits Retinoblastoma Progression by Modulating the miR-101-3p/STC1 Axis.Technol Cancer Res Treat. 2021 Jan-Dec;20:1533033821997831. doi: 10.1177/1533033821997831. Technol Cancer Res Treat. 2021. PMID: 33784880 Free PMC article.
-
Non coding RNAs as the critical factors in chemo resistance of bladder tumor cells.Diagn Pathol. 2020 Nov 12;15(1):136. doi: 10.1186/s13000-020-01054-3. Diagn Pathol. 2020. PMID: 33183321 Free PMC article. Review.
-
Role of non-coding RNAs and exosomal non-coding RNAs in retinoblastoma progression.Front Cell Dev Biol. 2022 Dec 23;10:1065837. doi: 10.3389/fcell.2022.1065837. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36619866 Free PMC article. Review.
-
Histone deacetylase (HDAC) 9: versatile biological functions and emerging roles in human cancer.Cell Oncol (Dordr). 2021 Oct;44(5):997-1017. doi: 10.1007/s13402-021-00626-9. Epub 2021 Jul 27. Cell Oncol (Dordr). 2021. PMID: 34318404 Free PMC article. Review.
-
miR-101-3p Serves as a Tumor Suppressor for Renal Cell Carcinoma and Inhibits Its Invasion and Metastasis by Targeting EZH2.Biomed Res Int. 2021 Jul 7;2021:9950749. doi: 10.1155/2021/9950749. eCollection 2021. Biomed Res Int. 2021. PMID: 34307682 Free PMC article.
References
-
- Busch M, Große-Kreul J, Wirtz JJ, Beier M, Stephan H, Royer-Pokora B, Metz K, Dünker N. Reduction of the tumorigenic potential of human retinoblastoma cell lines by TFF1 overexpression involves p53/caspase signaling and miR-18a regulation. Int J Cancer. 2017;141:549–560. doi: 10.1002/ijc.30768. - DOI - PubMed
-
- Chu WK, Law KS, Chan SO, Yam JC, Chen LJ, Zhang H, Cheung HS, Block NL, Schally AV, Pang CP. Antagonists of growth hormone-releasing hormone receptor induce apoptosis specifically in retinoblastoma cells. Proc Natl Acad Sci USA. 2016;113:14396–14401. doi: 10.1073/pnas.1617427113. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
