Efficacy and safety of mycophenolate mofetil for IgA nephropathy: An updated meta-analysis of randomized controlled trials
- PMID: 30186414
- PMCID: PMC6122311
- DOI: 10.3892/etm.2018.6418
Efficacy and safety of mycophenolate mofetil for IgA nephropathy: An updated meta-analysis of randomized controlled trials
Abstract
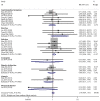
The efficacy and safety of mycophenolate mofetil (MMF) for immunoglobulin A nephropathy (IgAN) remains debatable. Therefore, the present meta-analysis was conducted with randomized controlled trials (RCTs). PubMed/MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials were analyzed to identify eligible trials. The pooled risk ratio (RR) with 95% confidence interval (CI) was estimated for all the dichotomous outcome measures. A total of eight RCTs with nine publications (n=510 patients) were included. No significant difference was noted between therapeutic regimens with and without MMF for renal remission and end stage renal disease (ESRD) of patients with IgAN (seven trials; RR, 1.250; 95% CI, 0.993-1.574; P=0.057; and four trials; RR, 0.728; 95% CI, 0.164-3.236; P=0.676). To further define the effects of MMF for renal remission, subgroup analysis was performed, demonstrating that MMF was significantly more effective compared with the placebo (three trials; RR, 2.152; 95% CI, 1.198-3.867; P=0.010), although the immunosuppressive regimens with MMF had no significantly different effects compared with those without MMF (four trials; RR, 1.140; 95% CI, 0.955-1.361; P=0.146), indicating that MMF was superior to placebo and had a similar efficacy to other immunosuppressants for renal remission. In addition, subgroup analysis for ESRD revealed no significant differences between MMF and placebo and between the immunosuppressive regimens with and without MMF (three trials; RR, 0.957; 95% CI, 0.160-5.726; P=0.962; and one trial; RR, 0.205; 95% CI, 0.010-4.200; P=0.303). Furthermore, there were no significant differences between the therapeutic regimens with and without MMF in terms of the risk of adverse events. The present meta-analysis demonstrated that MMF was more effective compared with the placebo, may have similar efficacy to other immunosuppressants in terms of inducing renal remission of IgAN and may not increase the risk of adverse events. The long-term effects of MMF on the prognosis of patients with IgAN require verification in further studies.
Keywords: end-stage renal disease; immunoglobulin A nephropathy; meta-analysis; mycophenolate mofetil; randomized controlled trial; renal remission.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
