Food-derived polyphenols inhibit the growth of ovarian cancer cells irrespective of their ability to induce antioxidant responses
- PMID: 30186979
- PMCID: PMC6121158
- DOI: 10.1016/j.heliyon.2018.e00753
Food-derived polyphenols inhibit the growth of ovarian cancer cells irrespective of their ability to induce antioxidant responses
Abstract
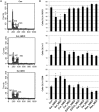
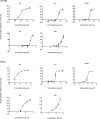
The use of plant polyphenols to prevent cancer has been studied extensively. However, recent findings regarding the cancer-promoting effects of some antioxidants have led to reservations regarding the therapeutic use of food-derived antioxidants including polyphenols. The aim of this study was to evaluate the therapeutic potential of food-derived polyphenols and their use and safety in cancer patients. The free-radical scavenging ability of sulforaphane and various food-derived polyphenols including curcumin, epigallocatechin gallate, epicatechin, pelargonidin, and resveratrol was compared with their growth inhibitory effect on ovarian cancer cells. Oxidative stress and/or antioxidant responses and anti-proliferative pathways were evaluated after administering sulforaphane and polyphenols at doses at which they have been shown to inhibit the growth of ovarian cancer cells. No correlation was observed between their ability to scavenge free radicals and their ability to inhibit the growth of ovarian cancer cells. With the exception of epigallocatechin gallate, all of the antioxidants that were tested at doses that inhibited cell growth significantly increased NAD(P)H quinone dehydrogenase I (NQO1) expression but induced cell cycle arrest and/or apoptotic signaling. Epigallocatechin gallate exhibited a higher free radical scavenging activity but did not induce NQO1 expression at either the mRNA or at the protein level. Treatment with polyphenols at physiological doses did not significantly alter the growth of ovarian cancer cells or NQO1 expression. Therefore, individual food-derived polyphenols appear to have different anti-cancer mechanisms. Their modes of action in relation to their chemical properties should be established, rather than collectively avoiding the use of these agents as antioxidants.
Keywords: Biochemistry; Cancer research; Cell biology; Food science.
Figures






References
-
- Key T.J., Schatzkin A., Willett W.C., Allen N.E., Spencer E.A., Travis R.C. Diet, nutrition and the prevention of cancer. Publ. Health Nutr. 2004;7(1A):187–200. - PubMed
-
- Collins A.R. Antioxidant intervention as a route to cancer prevention. Eur. J. Cancer. 2005;41(13):1923–1930. - PubMed
-
- Sayin V.I., Ibrahim M.X., Larsson E., Nilsson J.A., Lindahl P., Bergo M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014;6(221):221ra15. - PubMed
-
- Le Gal K., Ibrahim M.X., Wiel C., Sayin V.I., Akula M.K., Karlsson C. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015;7(308):308re8. - PubMed
-
- Tanvetyanon T., Bepler G. Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: a meta-analysis and evaluation of national brands. Cancer. 2008;113(1):150–157. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

