Role of genetic factors and ethnicity on the multiplicity of Plasmodium falciparum infection in children with asymptomatic malaria in Yaoundé, Cameroon
- PMID: 30186982
- PMCID: PMC6120745
- DOI: 10.1016/j.heliyon.2018.e00760
Role of genetic factors and ethnicity on the multiplicity of Plasmodium falciparum infection in children with asymptomatic malaria in Yaoundé, Cameroon
Abstract
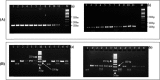
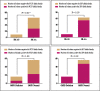
In this cross-sectional study, we investigated host genetic factors and ethnic variation in circulating Plasmodium falciparum merozoite surface protein 2 (msp-2) clones among children with asymptomatic malaria. Isolates from seventy two asymptomatic malaria children were used for genotyping block 3 of msp-2 gene by nested polymerase chain reaction (PCR). Sickle cell trait and glucose-6-phosphate dehydrogenase (G6PD) deficiency were analysed by restriction fragment length polymorphism of DNA products from PCR targeting codons 6 and 68 of the beta-globin (HBB) and G6PD genes respectively. ABO blood group was typed by agglutination method. A total of forty two msp-2 genotypes (20 for 3D7 and 22 for FC27) were detected for an average (standard error of mean) multiplicity of infection (MOI) of 2.45 (0.16). The MOI was statistically the same among the five identified ethnic groups (P = 0.83). The overall prevalence of sickle cell trait and G6PD deficiency were 12.50 % and 22.22 % respectively. MOI was similar between children with Hb AA and Hb AS genotypes (P = 0.42). MOI was significantly high among children with a mutant G6PD genotype (P = 0.017). MOI was significantly higher in blood group O than group A (P = 0.03). Our findings show that although ethnicity and sickle cell trait have no association with MOI, the association was observed with G6PD genotype and ABO group. The results suggest the need for extension and expansion of the current study in order to investigate the mechanisms involved.
Keywords: Clinical genetics; Epidemiology; Infectious disease; Pediatrics.
Figures

References
-
- White N.J., Pukrittayakamee S., Hien T.T., Faiz M.A., Mokuolu O.A., Dondorp A.M. Malaria. Lancet. 2014 Feb;383(9918):723–735. - PubMed
-
- Babiker H.A., Ranford-Cartwright L.C., Walliker D. Genetic structure and dynamics of Plasmodium falciparum infections in the Kilombero region of Tanzania. Trans. R. Soc. Trop. Med. Hyg. 1999 Feb;93(Suppl 1):11–14. - PubMed
-
- Wang B., Han S.-S., Cho C., Han J.-H., Cheng Y., Lee S.-K. Comparison of microscopy, nested-PCR, and Real-Time-PCR assays using high-throughput screening of pooled samples for diagnosis of malaria in asymptomatic carriers from areas of endemicity in Myanmar. J. Clin. Microbiol. 2014 Jun;52(6):1838–1845. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

