Valproic acid utilization among girls and women in Stockholm: Impact of regulatory restrictions
- PMID: 30187006
- PMCID: PMC6119758
- DOI: 10.1002/epi4.12228
Valproic acid utilization among girls and women in Stockholm: Impact of regulatory restrictions
Abstract
Objective: In November 2014, the European Medicines Agency (EMA) strengthened restrictions on the use of valproic acid in girls and women of childbearing potential. The objective of this study was to determine whether there has been a change in initiations of valproic acid treatment to females after the regulatory restrictions and to assess if such changes differed between indications (epilepsy and psychiatric disorder).
Methods: An interrupted time-series analysis was conducted using all initiations of valproic acid in Stockholm, Sweden. from January 2011 to June 2017. Female and male patients aged 0-45 years with a recorded diagnosis of epilepsy and/or a psychiatric disorder were compared.
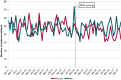
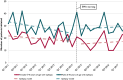
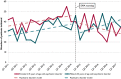
Results: Before the EMA warning, a decline in trend of valproic acid initiations was seen in patients with epilepsy. After the warning, a significant decrease of valproic acid initiations was seen in women with a psychiatric disorder, but not in women with epilepsy.
Significance: The regulatory warning appeared to have significantly influenced valproic acid initiations in women of childbearing age with a psychiatric disorder. No effect was seen in women with epilepsy, probably because the decline had started long before.
Keywords: Epilepsy; Interrupted time series; Psychiatric disorder; Regulatory warnings; Sex differences.
Figures
Similar articles
-
Impact of the EMA/FDA alerts on the use of effective contraceptive method in women of childbearing age undergoing valproic acid treatment in a long-stay psychiatric center.Epilepsy Behav. 2020 Jun;107:107072. doi: 10.1016/j.yebeh.2020.107072. Epub 2020 Apr 8. Epilepsy Behav. 2020. PMID: 32278266
-
Impact of recommendations on sodium valproate prescription among women with epilepsy: An interrupted time-series study.Epilepsy Behav. 2021 Dec;125:108449. doi: 10.1016/j.yebeh.2021.108449. Epub 2021 Nov 25. Epilepsy Behav. 2021. PMID: 34839242
-
Impact of regulatory restrictions on the use of valproic acid in women of childbearing age: An Italian study.Epilepsia. 2023 Apr;64(4):910-918. doi: 10.1111/epi.17526. Epub 2023 Feb 20. Epilepsia. 2023. PMID: 36727540
-
Alternatives to valproate in girls and women of childbearing potential with Idiopathic Generalized Epilepsies: state of the art and guidance for the clinician proposed by the Epilepsy and Gender Commission of the Italian League Against Epilepsy (LICE).Seizure. 2021 Feb;85:26-38. doi: 10.1016/j.seizure.2020.12.005. Epub 2020 Dec 21. Seizure. 2021. PMID: 33418162 Review.
-
Valproate in the treatment of epilepsy in girls and women of childbearing potential.Epilepsia. 2015 Jul;56(7):1006-19. doi: 10.1111/epi.13021. Epub 2015 May 16. Epilepsia. 2015. PMID: 25851171 Review.
Cited by
-
Mixed Impact of Direct Healthcare Professional Communications When Considering Proximal Outcomes and the Targeted Population: A Systematic Review.Pharmacoepidemiol Drug Saf. 2025 Mar;34(3):e70135. doi: 10.1002/pds.70135. Pharmacoepidemiol Drug Saf. 2025. PMID: 40122533 Free PMC article.
-
Valproate utilisation trends among women of childbearing potential in Ireland between 2014 and 2019: A drug utilisation study using interrupted time series.Pharmacoepidemiol Drug Saf. 2022 Jun;31(6):661-669. doi: 10.1002/pds.5427. Epub 2022 Mar 31. Pharmacoepidemiol Drug Saf. 2022. PMID: 35285110 Free PMC article.
-
Predictors of valproic acid steady-state serum levels in adult and pediatric psychiatric inpatients: a comparative analysis.Psychopharmacology (Berl). 2024 Sep;241(9):1883-1894. doi: 10.1007/s00213-024-06603-y. Epub 2024 May 11. Psychopharmacology (Berl). 2024. PMID: 38733528
-
Effectiveness of risk minimisation measures for valproate: A drug utilisation study in Europe.Pharmacoepidemiol Drug Saf. 2021 Mar;30(3):292-303. doi: 10.1002/pds.5166. Epub 2020 Nov 23. Pharmacoepidemiol Drug Saf. 2021. PMID: 33108674 Free PMC article.
-
Why Do Psychiatrists Still Prescribe Valproate to Women of Childbearing Potential?Front Psychiatry. 2020 Jul 24;11:739. doi: 10.3389/fpsyt.2020.00739. eCollection 2020. Front Psychiatry. 2020. PMID: 32848919 Free PMC article. No abstract available.
References
-
- Tomson T, Marson A, Boon P, et al. Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia 2015;56:1006–1019. - PubMed
-
- European Medicines Agency . Amendments to be included in sections of the summary of product characteristics for valproic acid/valproate containing medicinal products, as relevant, 2010. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/.... Accessed March 2017.
-
- Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol 2012;11:803–813. - PubMed
-
- Nadebaum C, Anderson V, Vajda F, et al. The Australian brain and cognition and antiepileptic drugs study: IQ in school‐aged children exposed to sodium valproate and polytherapy. J Int Neuropsychol Soc 2011;17:133–142. - PubMed
-
- Bromley RL, Mawer G, Love J, et al. Early cognitive development in children born to women with epilepsy: a prospective report. Epilepsia 2010;51:2058–2065. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

