Peptide supramolecular materials for therapeutics
- PMID: 30187042
- PMCID: PMC6188840
- DOI: 10.1039/c7cs00735c
Peptide supramolecular materials for therapeutics
Abstract
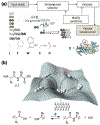
Supramolecular assembly of peptide-based monomers into nanostructures offers many promising applications in advanced therapies. In this Tutorial Review, we introduce molecular designs to control the structure and potential biological function of supramolecular assemblies. An emphasis is placed on peptide-based supramolecular nanostructures that are intentionally designed to signal cells, either directly through the incorporation of amino acid sequences that activate receptors or indirectly by recruiting native signals such as growth factors. Additionally, we describe the use and future potential of hierarchical structures, such as single molecules that assemble into nanoscale fibers which then align to form macroscopic strings; the strings can then serve as scaffolds for cell growth, proliferation, and differentiation.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

