Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: A multidisciplinary position paper
- PMID: 30187496
- PMCID: PMC6585802
- DOI: 10.1002/hon.2540
Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: A multidisciplinary position paper
Abstract
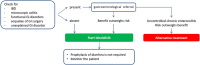
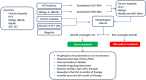
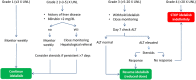
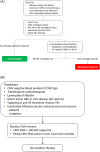
The introduction of new therapeutic agents in chronic lymphocytic leukemia (CLL) and follicular lymphoma (FL), including the new kinase inhibitor idelalisib, has changed the therapeutic landscape of these diseases. However, the use of idelalisib is associated with a peculiar profile of side effects, which require an optimization of the current approach to prophylaxis and supportive treatment. Moving from the recognition that the abovementioned issue represents an unmet need in CLL and FL, a multidisciplinary panel of experts was convened to produce a consensus document aiming to provide practical recommendations for the management of the side effects during idelalisib therapy for CLL and FL. The present publication represents a consensus document from a series of meetings held during 2017. The Panel generated clinical key questions using the criterion of clinical relevance through a Delphi process and explored 4 domains, ie, diarrhea/colitis, transaminitis, pneumonitis, and infectious complications. Using the consensus method, the Panel was able to shape recommendations which may assist hematologist to minimize adverse events and guarantee adherence to treatment in patients with CLL and FL candidate to receive idelalisib.
Keywords: adverse events; chronic lymphocytic leukemia; follicular lymphoma; idelalisib.
© 2018 The Authors Hematological Oncology Published by John Wiley & Sons Ltd.
Conflict of interest statement
Funding of the project was provided from at‐arm's‐length contribution from Gilead (Italy). The funding source had no role in identifying statements, abstracting data, synthesizing results, or preparing the manuscript or in the decision to submit the manuscript for publication.
Figures


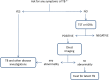
References
-
- Wiestner A. Emerging role of kinase‐targeted strategies in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2012;2012:88‐96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

