Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model
- PMID: 30188509
- PMCID: PMC6300528
- DOI: 10.1038/s41386-018-0185-7
Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model
Abstract
The 2017 American College of Neuropychopharmacology (ACNP) conference hosted a Study Group on 4 December 2017, Establishing best practice guidelines to improve the rigor, reproducibility, and transparency of the maternal immune activation (MIA) animal model of neurodevelopmental abnormalities. The goals of this session were to (a) evaluate the current literature and establish a consensus on best practices to be implemented in MIA studies, (b) identify remaining research gaps warranting additional data collection and lend to the development of evidence-based best practice design, and (c) inform the MIA research community of these findings. During this session, there was a detailed discussion on the importance of validating immunogen doses and standardizing the general design (e.g., species, immunogenic compound used, housing) of our MIA models both within and across laboratories. The consensus of the study group was that data does not currently exist to support specific evidence-based model selection or methodological recommendations due to lack of consistency in reporting, and that this issue extends to other inflammatory models of neurodevelopmental abnormalities. This launched a call to establish a reporting checklist focusing on validation, implementation, and transparency modeled on the ARRIVE Guidelines and CONSORT (scientific reporting guidelines for animal and clinical research, respectively). Here we provide a summary of the discussions in addition to a suggested checklist of reporting guidelines needed to improve the rigor and reproducibility of this valuable translational model, which can be adapted and applied to other animal models as well.
Conflict of interest statement
The authors declare no competing interests.
Figures
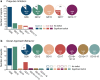
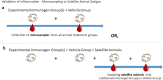
References
-
- Brown AS, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–80. - PubMed
-
- Machon RA, Mednick SA, Huttunen MO. Fetal viral infection and adult schizophrenia—empirical findings and interpretation. Neural Dev Schizophr. 1995;275:191–202.
-
- Brown AS, et al. A.E. Bennett Research Award. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49:473–86. - PubMed
Publication types
MeSH terms
Grants and funding
- P50 MH094268/MH/NIMH NIH HHS/United States
- F31 MH090749/MH/NIMH NIH HHS/United States
- R01 ES028125/ES/NIEHS NIH HHS/United States
- R01 MH083728/MH/NIMH NIH HHS/United States
- P51 OD011107/OD/NIH HHS/United States
- U54 HD079125/HD/NICHD NIH HHS/United States
- R01 NS060125/NS/NINDS NIH HHS/United States
- R01 DA041208/DA/NIDA NIH HHS/United States
- P50 MH106438/MH/NIMH NIH HHS/United States
- R01 ES019004/ES/NIEHS NIH HHS/United States
- R15 MH114035/MH/NIMH NIH HHS/United States
- R01 ES025549/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

