Alterations of the nigrostriatal pathway in a 6-OHDA rat model of Parkinson's disease evaluated with multimodal MRI
- PMID: 30188909
- PMCID: PMC6126820
- DOI: 10.1371/journal.pone.0202597
Alterations of the nigrostriatal pathway in a 6-OHDA rat model of Parkinson's disease evaluated with multimodal MRI
Abstract
Parkinson's disease is characterized by neurodegeneration of the dopaminergic neurons in the substantia nigra pars compacta. The 6-hydroxydopamine (6-OHDA) rat model has been used to study neurodegeneration in the nigro-striatal dopaminergic system. The goal of this study was to evaluate the reliability of diffusion MRI and resting-state functional MRI biomarkers in monitoring neurodegeneration in the 6-OHDA rat model assessed by quantitative histology. We performed a unilateral injection of 6-OHDA in the striatum of Sprague Dawley rats to produce retrograde degeneration of the dopamine neurons in the substantia nigra pars compacta. We carried out a longitudinal study with a multi-modal approach combining structural and functional MRI together with quantitative histological validation to follow the effects of the lesion. Functional and structural connectivity were assessed in the brain of 6-OHDA rats and sham rats (NaCl injection) at 3 and 6 weeks post-lesioning using resting-state functional MRI and diffusion-weighted. Our results showed (i) increased functional connectivity in ipsi- and contra-lesioned regions of the cortico-basal ganglia network pathway including the motor cortex, the globus pallidus, and the striatum regions at 3 weeks; (ii) increased fractional anisotropy (FA) in the ipsi- and contralateral striatum of the 6-OHDA group at 3 weeks, and increased axial diffusivity (AD) and mean diffusivity in the ipsilateral striatum at 6 weeks; (iii) a trend for increased FA in both substantia nigra of the 6-OHDA group at 3 weeks. Optical density measurements of tyrosine-hydroxylase (TH) staining of the striatum showed good correlations with the FA and AD measurements in the striatum. No correlations were found between the number of TH-stained dopaminergic neurons and MRI measurements in the substantia nigra. This study suggested that (i) FA and AD were reliable biomarkers to evaluate neurodegeneration in the cortico-basal ganglia network of the 6-OHDA model, (ii) diffusion MRI and resting-state functional MRI (rsfMRI) were not sensitive enough to detect changes in the substantia nigra in this model.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
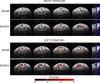

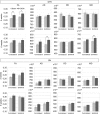

References
-
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20(4):415–455. - PubMed
-
- Lehéricy S, Vaillancourt DE, Seppi K, Monchi O, Rektorova I, Antonini A, et al. ; International Parkinson and Movement Disorder Society (IPMDS)-Neuroimaging Study Group. The role of high-field magnetic resonance imaging in parkinsonian disorders: Pushing the boundaries forward. Mov Disord. 2017;32(4):510–525. 10.1002/mds.26968 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

